Disinfectant Evaluation

Intended Learning objectives
At the end of this lecture, student will be able to
• Classify different methods for disinfectant evaluation
• Differentiate between bactericidal and bacteriostatic evaluation tests
• Explain the various bacteriostatic evaluation tests for disinfectants
• Explain the significance of capacity tests
• Explain the method for evaluation of solid disinfectants
Introduction
• Disinfectant evaluation – Tests used to assess bacteriostatic and bactericidal activity of disinfectants
• Bacteriostatic activity – ability of an disinfectant to stop the growth of microorganisms
• Bactericidal activity – ability to kill the organism
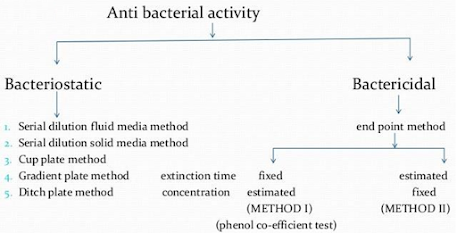
Tests for disinfectant activity
| SUBSTANCE TESTED | BACTERIOSTATIC TESTS | BACTERICIDAL TESTS |
| Liquid disinfectants | Serial dilution in fluid media | End-point or extinction time methods |
| Serial dilution in solid media | Counting methods | |
| Cup-plate, fish-spine bead and filter paper methods | Turbidometric assessment | |
| Gradient-plate method | ‘In use’ and other tests | |
| Ditch-plate technique | Additional in vivo tests can be applied | |
| Semi-solid antibacterial formulations, e.g. creams, ointments, pastes and gels | Cup-plate methods | Modified end-point or extinction time methods |
| The ditch-plate technique | Additional in vivo tests can be applied (e.g. skin tests) | |
| Solid disinfectants, disinfectant powders | Inhibition on seeded agar | Modified end-point or extinction time methods |
| Aerial disinfectants | Use of slit-sampler in test chamber | |
Tests applied to liquid disinfectants Bacteriostatic activity
Serial dilution in fluid media
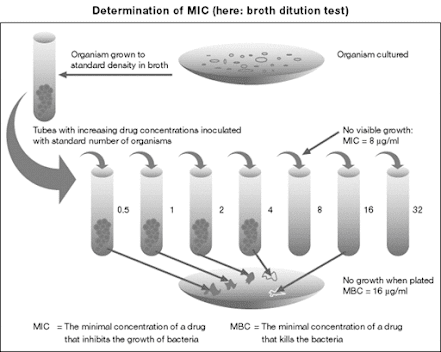
• Minimum Inhibitory Concentration (MIC) – the minimum concentration preventing detectable growth is taken as the measurement of bacteriostatic activity
• MIC varies with inoculum size, medium used and incubation conditions.
Serial dilution in solid media
1. A suitable volume of double strength nutrient agar
2. Diluted with an equal volume of bacteriostatic solution
3. Poured into sterile petri plate
4. Surface is dried by incubating at 37⁰C for 1 h
5. Drops of the test organism are placed on the dried surface
6. Incubated for 2 to 3 days
7. A separate petri dish is used for each concentration of bacteriostatic
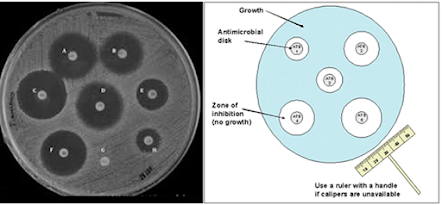
• Cup-plate, fish-spine and filter paper methods
• Agar medium is melted, cooled suitably and inoculated with the test organism
• Poured into a sterile petri dish
• Cup-plate: holes about 8 mm in diameter are cut in the agar with the help of a sterile cork borer
• Antimicrobial agent is placed in the holes
• Fish-spine bead, filter paper and cylinder method
• In all the cases, zones of inhibition may be observed
• The diameter of zones of inhibition gives rough indication of
– The relative activities of different antimicrobial substances against the test organism
– The effect of different concentrations of antimicrobial substance
The gradient – plate method
• Agar is streaked in the same line as the slope of the agar (along the concentration gradient) and reincubated.
• Approximate MIC is obtained from the equation
MIC=CX(x/y)mg/ml
C= concentration, in mg/ml, in total volume (i.e, volume of wedges A and B)
X=Length of growth, in cm
Y= total length of possible growth, in cm
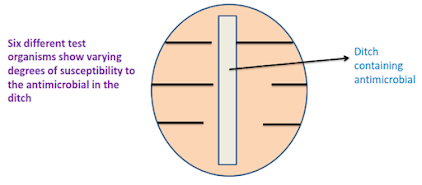
The ditch-plate technique
Carrier test
• A carrier such as a silk or catgut thread is contaminated by submersion in a liquid culture of the test organism
• The carrier is then dried and brought in contact with the disinfectant for a given exposure time
• Cultured in a nutrient broth
• No growth indicates activity of the disinfectant tested whereas growth indicates a failing.
• By multiplying the test concentrations of the disinfectant and the contact times, a potentially active concentration-time relationships of the disinfectant is obtained.
Limitations
The number of bacteria dried on a carrier is hard to standardize
The survival of the bacteria on the carrier during drying is not constant.
The AOAC Use – Dilution Test
• The AOAC Use-dilution test
• AOAC (American Association of Official Analytical Chemists)
• A carrier-based test
• Organisms: Salmonella cholerasuis, S. aureus and P. aeruginosa
• Carriers (stainless steel cylinders) are meticulously cleaned, sterilized, cooled and inoculated with a test organism by immersing in one of the culture suspensions
• The cylinders are drained on filter paper, dried at 37°C for 40 minutes, exposed to the use-dilution of the disinfectant for 10 minutes.
• After transfer from the disinfectant, the treated test surfaces are incubated in the neutralizing growth medium for 48 hours
• The number of tubes showing growth of the target microorganism is recorded.
• To “PASS” a 60 carrier test, at least 59 of the 60 surfaces tested must demonstrate complete disinfection (no detectable growth of the target microorganism in the test tubes containing neutralizing growth medium)
• To “PASS” a 10 carrier test, complete disinfection must take place on all test surfaces.
Suspension tests
• A sample of the bacterial culture is suspended into the disinfectant solution
• After exposure it is verified by subculture whether this inoculum is killed or not.
• Suspension tests are preferred to carrier tests as the bacteria are uniformly exposed to the disinfectant.
Types
A. Qualitative suspension tests:
• Loopful of bacterial suspension brought into contact with the disinfectant
• A loopful of this mixture cultured for surviving organisms.
• Results expressed as ‘growth’ or ‘no growth’.
B. Quantitative suspension tests.
• The number of surviving organisms (B) is counted and compared to the original inoculum size (A).
Microbicidal effect (ME) = Log (A) – Log (B)
• ME = 1 → killing of 90% of the initial number
• ME = 2 → 99% killed.
• A generally accepted requirement is:
• ME ≥ 5 →99.999% of the germs are killed.
Capacity test
• The ability to retain activity in the presence of an increasing load is the capacity of the disinfectant.
• In a capacity test, the disinfectant is challenged repeatedly by successive additions of bacterial suspension until its capacity to kill has been exhausted.
• Capacity tests simulate the practical situations of housekeeping and instrument disinfection.
• Best known capacity test is the Kelsey-Sykes test (Kelsey and Sykes, 1969).
Kelsy-Sykes ‘In-use’ tests
• A triple challenge test, designed to determine concentrations of disinfectant that will be effective in clean and dirty conditions.
• Organisms: 4 organisms (S. aureus, E.coli, Psedomonas aeruginosa and Proteus vulgaris)
• Three successive loads of bacteria (additions) Transfer 1ml at 0, 10, and 20 mins
• Temp: 20⁰C
• Calibrated pipette for subculture rather than loop
• Clean and dirty conditions
• Assessment (kill or not)
Time from start (min)
| Procedure |
0 | Inoculate 3ml of the disinfectant dilution with 1ml of bacterial suspension in broth, yeast or serum and shake gently (this gives a bactericide/bacteria reaction mixture) |
8 | Remove sample from above reaction mixture with a 50 dropper pipette. Transfer 1 drop to each of 5 tubes of liquid recovery media, or 5 drops to the surface of a nutrient agar plate |
10 | To bactericide or bacteria reaction mixture, prepared at start (time 0), add a second 1 ml of bacterial suspension |
• Sets that contain two or more negative cultures are recorded as a negative result.
• The disinfectant passes at the dilution tested if negative results are obtained after the first and second challenges.
• The third challenge is not included in the pass/fail criterion but positive cultures serve as inbuilt controls.
• If there are no positive cultures after the third challenge, a lower concentration of the disinfectant may be tested.
Tests on aerial disinfectants
• A closed room of approximately cubic dimensions and 1000cu ft capacity is used
• Fans incorporated to ensure uniform mixing of bacteria and bactericide
• Staphylococcus albus (non clumping strain) is used as the test organism
• Dispersion of organism is done using collision inhaler
• Air samples are taken using a slit sampler at suitable intervals
• The room should be initially free from extraneous microorganisms
• Temperature and humidity of air controlled
• Cyclopentanol-1-carboxylic acid is chosen as the reference standard for air disinfection
Tests applied on solid disinfectants
• Disinfectant powder is mixed with an inert substance such as talc or kieselguhr to form a disinfectant powder
• Powder is dusted onto inoculated plates
• Inert diluent is used as the control
• Alternatively Rideal Walker coefficient can also be used
• A weighed sample is shaken with distilled water at 18⁰C for 3 min
• The suspension is used for the Rideal-walker test
Summary
• Bacteriostatic tests – evaluate the property of stopping the growth of microorganisms
• Bactericidal tests – evaluate the killing property
• Bactericidal tests
– Serial dilution in solid media
– Serial dilution in liquid media
– Cup plate
– Ditch plate
– Gradient plate
• Aerial disinfectants evaluated by using a slit sampler and Staph albus as test organism
• Solid disinfectants – sprayed over the surface of a inoculated plate
Disinfectant Evaluation FAQs
Q1: What is the primary goal of disinfectant evaluation?
The primary goal of disinfectant evaluation is to determine the effectiveness of a disinfectant in killing or inhibiting the growth of microorganisms.
Q2: Are there specific methods for evaluating aerial disinfectants?
Yes, the gradient plate method is a common technique for assessing the effectiveness of aerial disinfectants.
Q3: How is the zone of inhibition measured in the cup plate method?
The zone of inhibition in the cup plate method is measured by assessing the area where bacterial growth is inhibited around the disinfectant-soaked filter paper disc.
Q4: Why is safety evaluation essential in disinfectant testing?
Safety evaluation ensures that disinfectants do not pose risks to humans, animals, or the environment.
Q5: Can the same evaluation method be used for all disinfectants?
The choice of evaluation method depends on the specific disinfectant, the microorganisms it targets, and its intended use. Different disinfectants may require different evaluation techniques.
For Disinfectant Evaluation PDF Notes Click on Download Button