Acid Base Theory
Learning Objectives
At the end of this lecture, the student will be able to:
Classify:
• Acids
• Bases and
• Salts based on various theories
History of Acids and Bases
In the early days of chemistry chemists were organizing
physical and chemical properties of substances. They discovered that many substances could be placed in two different property categories:
Substance A | Substance B |
1. Sour taste | 1. Bitter taste |
2. Reacts with carbonates to make CO2 | 2. Reacts with fats to make soaps |
3. Reacts with metals to produce H2 | 3. Do not react with metals |
4. Turns blue litmus pink | 4. Turns red litmus blue |
5. Reacts with B substances to make salt and water | 5. Reacts with A substances to make salt and water |
6. pH < 7 | 6. pH >7 |
Arrhenius was the first person to suggest a reason why substances are in A or B due to their ionization in water
Arrhenius Theory
The Swedish chemist Svante Arrhenius proposed the first
definition of acids and bases (1887)
According to the Arrhenius model:
“Acids are substances that dissociate in water to produce H+ ions and bases are substances that dissociate in water to produce OH– ions”
NaOH (aq) > Na+ (aq) + OH–(aq) Base
HCl (aq) > H+ (aq) + Cl–(aq) Acid
Arrhenius theory: Neutralization reactions
• Arrhenius acids and bases react with each other to form
water and aqueous salts in neutralization reactions
H+ (aq) + A–(aq) + M+ (aq) + OH–(aq) > H2O (l) + M+ (aq) + A–(aq)
• The net ionic equation is
H+ (aq) + OH– (aq) > H2O (l)
Classification of acid and base based on an Arrhenius concept:
ACID | BASE |
Strong acid | Strong base |
weak acid | Weak base |
Mono basic acid | Mono acidic base |
Dibasic acid | Di acidic base |
Tribasic acid | Tribasic base |
Limitations of Arrhenius concept
• The definitions are only in terms of aqueous solution and
not in terms of substance
• The theory is not able to explain acidic and basic properties of substances in non-aqueous solvents
Example: Ammonium nitrate in liquid ammonia acts as an acid though it does not give H+ ions
• The basic nature of substances like ammonia or sodium
carbonate which do not contain OH– ions was not explained by this concept
• The acidic nature of carbon di oxide, sulphur di oxide
which do not contain H+ ions was not explained by this concept
• The neutralisation of acid and base in absence of solvent
could not be explained
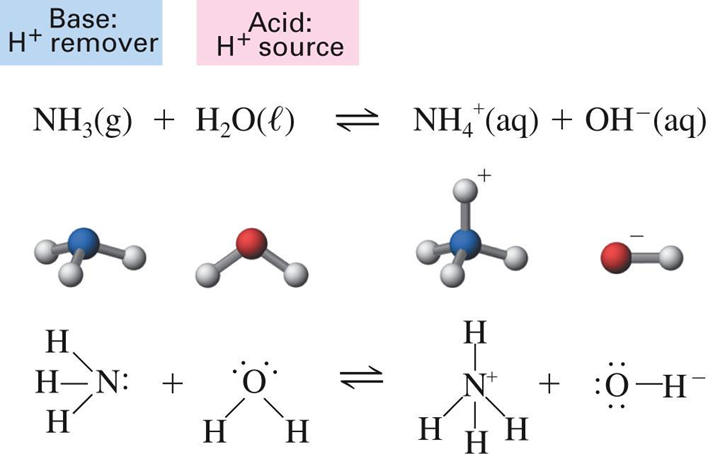
Bronsted Lowry Theory (1923)
Johannes Bronsted and Thomas Lowry revised Arrhenius’s
acid-base theory to include this behavior. They defined acids and bases as follows:
“An acid is a hydrogen containing species that donates a
proton. A base is any substance that accepts a proton”
HCl (aq) + H2O (l) > Cl–(aq) + H3O+(aq)
In the above example what is the Brønsted acid? What is the
Brønsted base?
In reality, the reaction of HCl with H2O is an equilibrium and occurs in both directions, although in this case the equilibrium lies far to the right.
HCl (aq) + H2O (l) > Cl–( aq) + H3O+ (aq)
For the reverse reaction Cl– behaves as a Bronsted base and H3O+ behaves as a Bronsted acid.
The Cl- is called the conjugate base of HCl. Bronsted acids and bases always exist as conjugate acid-base pairs.
Bronsted-Lowry Theory of Acids & Bases Conjugate Acid-Base Pairs
General Equation
Bronsted-Lowry Theory of Acids & Bases: Example
Notice that water is both an acid & a base = amphoteric
Bronsted-Lowry Theory of Acids & Bases Proton transfer reactions
• Pairs of compounds are related to each other through
Bronsted-Lowry acid-base reactions. These are conjugate acid-base pairs.
• Generally, an acid HA has a conjugate base A– (a proton hastransferred away from the acid). Conversely, a base B has a conjugate acid BH+ (a proton has transferred toward the base).
Classification:
Bronsted acid:
Mono protic acid: Capable of donating one proton only
Example: HF, CH3COOH
Poly protic acid: Capable of donating more than one proton
Example: H2S, H2O
Bronsted Base:
Mono protic base: Which can accept one proton
Example: water
Poly protic base: Which can accept two or more proton
Example: Sulphate ion, Phosphate ion
Lewis acids and bases
Gilbert Newton Lewis (1875-1946) influential American
chemist. His theories include the Lewis dot structure taught in Chem120 and covalent bond theories.
Lewis acids are electrophils: H+, Na+, BF3,
Lewis bases are nucleophils: NH3, H2O, PH3
Acid base reactions:
BF3 + :NH3 > F3B:NH3
Lewis acids and bases
In general LA + :LB > LA-LB
Lux flood theory
• It is applicable only for oxide system
According to this theory:
• A base is an oxide ion donor and
• An acid is an oxide ion acceptor
Example: CaO + SiO2 → CaSiO3
Uranvonish theory
This theory is given by Russian chemist: Uranvonish in 1939
• An acid is a substance which reacts with a base to give up
a cation
• A base is a substance that react with an acid giving up an
anion and accepts cation or electron
Example: SO3— + Na2O → Na2SO4
Summary
Acids:
• Acids are sour tasting
• Arrhenius acid: Any substance that, when dissolved in water, increases the concentration of hydronium ion (H3O+)
• Bronsted-Lowry acid: A proton donor
• Lewis acid: An electron acceptor
Bases:
• Bases are bitter tasting and slippery
• Arrhenius base: Any substance that, when dissolved in water, increases the concentration of hydroxide ion (OH–)
• Bronsted-Lowery base: A proton acceptor
• Lewis acid: An electron donor
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf