Formulation of Emulsions

Learning Objectives
At the end of this lecture, student will be able to
• Explain the uses of emulsions
• Explain the formulation of emulsions
• Classify emulsifying agents
• Describe the properties of various emulsifying agents
• Explain the steps involved in the preparation of emulsions
• Outline the primary emulsion formulae
• Describe different methods of preparation of emulsions
Emulsions
Emulsions, at their core, are stable mixtures of two or more liquids that would not naturally combine. Typically, they consist of two primary phases: a continuous phase (often water) and a dispersed phase (usually oil). Emulsifying agents are essential components that facilitate the stable coexistence of these immiscible liquids. These agents reduce surface tension and prevent the substances from separating over time.
Routes of Administration of Emulsions
1) Oral Emulsions
E.g 1: Liquid Paraffin Oral Emulsion
E.g 2: Castor oil Emulsion
E.g 3: Cod-Liver oil Emulsion
2) Rectal Emulsions: Enemas as O/W emulsions.
E.g 1: Starch Enema
3) Topical Emulsions: For external use – emulsions may be O/W or W/O
E.g 1: Turpentine liniment IP
E.g 2: Oily Calamine lotion BPC
4) Parenteral emulsions: To be injected
E.g. Intravenous Fat emulsion
Formulation of Emulsions
1. Choice of emulsion type
2. Choice of oil phase
3. Choice of emulsifying agent (emulgent)
1. Choice of emulsion type
– Fats or oils for oral administration – O/W emulsions.
– I.V administration – O/W
– I.M injections – W/O emulsion – if a water soluble drug – for depot therapy.
– Semisolid emulsions for external application – O/W or W/O
-Topical application of water soluble drugs- O/W
– Cleansing the skin of oil soluble dirt- W/O
2. Choice of oil phase
• Oil phase of an emulsion is the active agent – its conc. in the product is predetermined.
• E.g. Liquid paraffin, castor oil, cod liver oil and arachis oil – formulated as emulsions for oral administration.
• Cottonseed oil, soya bean oil and safflower oil – are used in parenteral emulsions -their high calorific value.
• Turpentine oils, benzyl benzoate – external application
• Liquid paraffin, hard/soft paraffin – used alone or in combination to control emulsion consistency
3. Choice of emulsifying agent (emulgent)
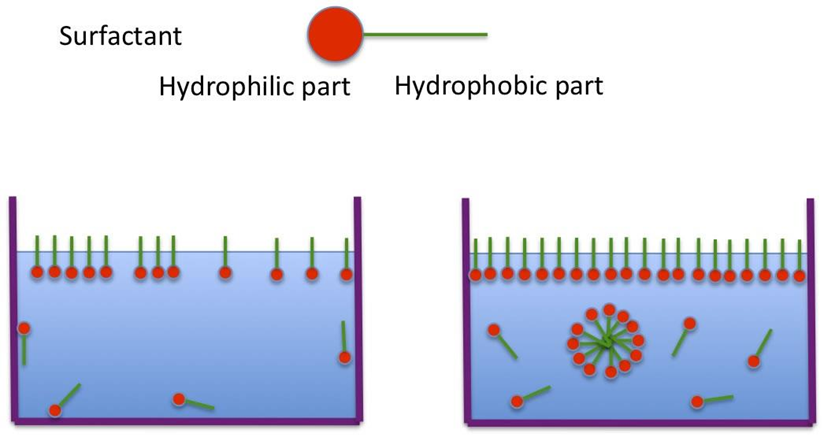
• Emulsifying agents / Emulgents/ emulsifiers
• Prevent the coalescence of the globules of the dispersed phase.
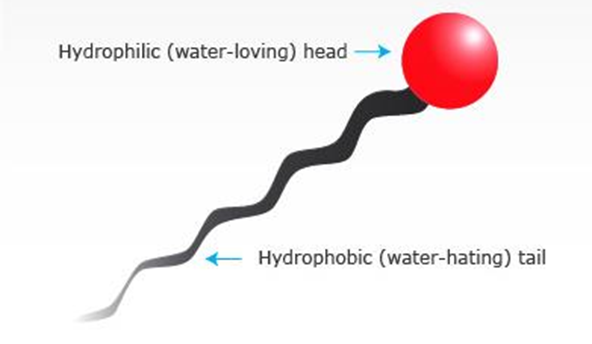
• They have both a hydrophilic and a lipophilic part in their chemical structure.
• Adsorbed onto the oil/water interface
• Provide a protective barrier around the dispersed droplets.
• Reduce the interfacial tension between oil phase & aqueous phase
• Increases miscibility
• Forms a stable emulsion.
Representation of action of Emulgent
It is a thick, creamy sauce often used as a condiment. It is a stable emulsion of oil, egg yolks and either vinegar or lemon juice
To solubilize oil soluble drugs
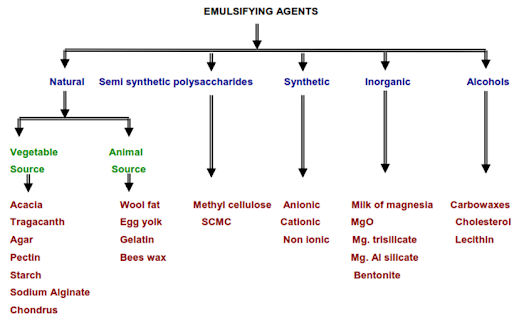
Classification of Emulsifying Agents
Natural Emulsifying Agents
1. Acacia
• Best emulsifying agent
• For extemporaneous preparation of emulsion
• Good quality, stability & appearance – achieved with a mortar & pestle
• Concentrated emulsion in the initial stage of preparation is viscous & sticky.
• Due to vigorous shearing action of the pestle- oil is easily reduced to fine globules)
• Emulsions are of low viscosity
• Thickening agents such as Tragacanth & sodium alginate should be added.
• Palatable and stable over a wide pH range (2 – 1 0).
2. Tragacanth
• Not used alone because of its high viscosity
• The emulsions are coarse
• Used as a stabilizer in acacia emulsions
• Used in proportion 1: 10(acacia).
3. Sodium alginate
• From brown seaweed
• High viscosity
• Used as an emulsion stabilizer in acacia emulsions
4. Agar
– Dried extract from certain seaweeds
– Emulsion stabilizer in acacia emulsion
– Soluble in boiling water
– High viscosity
5. Starch
– Poor emulsifying agent
-Preparation of enemas containing oils
6. Pectin
– Obtained from the inner rind of citrus fruits or from apple pulp
– Good O/W emulgent
– Degrades in alkaline pH
– Used as a stabilizer in cosmetic creams & lotions.
7. Chondrus (Irish Moss or Carrageen)
– Dried seaweed
– Not suitable for small scale emulsification
– Time consuming
– Used in cod liver oil emulsions
– Masks the unpleasant odour & taste of the oil.
– 2.5% mucilage – emulsify an equal volume of fixed oil.
8. Wool fat (lanolin)
– Wax from the sebaceous glands of sheep
– Consists of fatty acid esters of cholesterol + other sterols + normal fatty alcohols.
– Can absorb 50% of water
– When mixed with other fatty substances it can emulsify several times its own weight of aqueous or hydroalcoholic liquids.
– The emulsions will be of W/O type
9. Gelatin
– From skin and bones of animals
– Used for the emulsification of liquid paraffin emulsions at a concentration of 1 %
10. Egg yolk
– Emulsifying agent – lecithin & cholesterol
– Rarely used in industrial preparations
– Spoiled during transportation –
– Good preservation is necessary
11. Bees Wax
– Natural wax produced in the bee hive of honey bees
– Female worker bees have wax producing glands
– The wax is used to build honey comb cells
Semi synthetic polysaccharides
1. Methyl cellulose
– Low Viscosity grades are used
– Emulgents & emulsion stabilizers
– Suitable for emulsifying mineral & vegetable oils –
– Concentration of 2 %
2. SCMC
– Medium viscosity grades are used
– Concentration of 0.5 -1 %
– Emulsion stabilizers.
Synthetic EA
1. Anionic
– In aqueous solution they ionize into a large anion and a small cation
– This anion has emulsifying ability
– They bear a negative charge
– There are of 5 types:
* Alkali metal & ammonium soaps
* Soaps of divalent & trivalent metals
* Amine soaps
* Alkyl sulphates
* Alkyl phosphates
Alkali Soap Emulsions (Monovalent soaps)
• Na+, K+ and NH4+soaps
• Stable above pH 10
• Sensitive to acids
• High concentration of electrolytes can salt out the soap
• Incompatible with polyvalent cations (Mg2+, Al3+, Zn2+)
• Cations cause phase reversal.
• Physiological action & unpleasant taste
• Unsuitable for internal emulsions
• High alkaline pH- avoid usage on broken skin.
• Emulgents only in O/W emulsions
E.g. Sodium stearate, Potassium stearate, Ammonium stearate
Soaps of Divalent & Trivalent Metals
• Calcium soaps (Calcium stearate) are preferred
• Used as W/O emulsifying agents
• Cannot be used internally
• Less alkaline & less sensitive to acid
• Incompatible with monovalent soaps
Triethanolamine (amine soaps)
• Neutral (pH 7.5 to 8)
• Produce O/W emulsions
• Can be applied to broken skin
• Unsuitable for internal use.
Alkyl Sulphates
• Esters of fatty alcohols and sulphuric acid
• SLS is preferred – O/W emulsions
• Low stability
• Can be used with fatty alcohols- to increase stability
Alkyl Phosphates
• Used in combination with fatty alcohols
• Similar to alkyl sulphate
• The alcohols groups are phosphated instead of sulphated
2. Cationic
• In aqueous solution they ionize into a large cation and a small anion
• This cation has emulsifying ability
• They bear a positive charge
E.g. Quarternary ammonium compounds.
• Emulgent, disinfectant & preservative properties
• Combined with fatty alcohols for better emulsification
E.g. Benzalkonium chloride, Benzethonium chloride & cetrimide (Cetyl trimethyl ammonium bromide).
3. Non- ionic:
• They do not ionize in aqueous solution
• The emulsion will be stable over a wide range of pH
• Not affected by addition of acids & electrolytes
E.g. Glycol & Glycerol esters (Glyceryl monostearate), Sorbitan esters (Spans), Polysorbates (Tweens), Macrogols (Polyethylene glycol), Poly vinyl alcohol.
Non- Ionic Surfactants
Spans (Sorbitan Esters) | Tweens (Polysorbates) |
Span 20 – lauric acid | Tween 20 – lauric acid |
Span 40- palmitic acid | Tween 40- palmitic acid |
Span 60 – stearic acid | Tween 60 – stearic acid |
Span 80 – oleic acid | Tween 80 – oleic acid |
Inorganic agents
• Finely divided solids
• Balanced hydrophobic & hydrophillic properties
• If the solid particles are preferentially wetted by the oil- then W/O emulsions
• If wetted by water – O/W emulsions
E.g. Milk of magnesia (10-20%) Magnesium oxide (5-10%) Magnesium aluminium silicate (1%)
Alcohols
1. Carbowaxes
– Used in ointments & creams
– If Mol. weight is between 200-700 – viscous, light coloured, hygroscopic liquids.
– Mol. weight above 1000 – wax like solids.
2. Cholesterol
– Used only in combination with other emulsifying agents
3. Lecithin
– Yellowish brown fatty substance
– occurs in plant and animal tissues
– Isolated from egg yolk, bile, human brain tissue, fish roe, chicken and sheep brain, soy beans, eggs, cotton seed and sunflower.
Preparation of Emulsions
The preparation of an emulsion involves two stages:
1. Preparation of the primary emulsion
2. Dilution of the primary emulsion.
Calculation of Primary Emulsion Formula
Type of oil | Examples | Oil | Water | Gum |
Fixed oil | Arachis oil, Castor oil, Cod liver oil, Almond oil | 4 | 2 | 1 |
Mineral oil | Liquid Paraffin | 3 | 2 | 1 |
Volatile(aromatic) oil | Cinnamon oil, Turpentine oil, Peppermint oil | 2 | 2 | 1 |
Oleo resin | Male fern extract | 1 | 2 | 1 |
Methods for Preparation of Emulsions
1) Trituration method
a) Dry Gum or Continental method: the emulsifying agent is mixed with the oil before the addition of water.
b) Wet Gum or English method: the emulsifying agent is added to the water to form mucilage and then the oil is slowly incorporated to form the emulsion.
2) Bottle or Forbes method:
– Preparing emulsions containing volatile and other non-viscous oils.
– Both dry gum and wet gum methods can be employed
Formulation of Emulsions Summary
1. Routes of administration of emulsions – Oral, Topical, Rectal, Parenteral
2. Formulation of emulsions
– Choice of emulsion type: O/W or W/O
– Choice of oil phase: Depending on whether internal/external/parenteral
– Choice of emulsifying agent (Emulgent)
3. Emulsifying agents: Are of 5 types
– Natural (Plant/ Animal source)
– Semi synthetic polysaccharides
– Synthetic
– Inorganic
– Alcohols
4. Emulsifying agents obtained from plant sources- Acacia, Tragacanth, Sodium Alginate, Agar, Pectin, Chondrus, and Starch
5. Emulsifying agents obtained from animal sources- Beeswax, Wool Fat, Gelatin and Egg Yolk
6. Semi- Synthetic emulsifying agents: Cellulose derivatives
7. Synthetic emulsifying agents: Anionic, Cationic and Non- ionic
8. Steps involved in the preparation of emulsions- Primary emulsion preparation and Dilution of Primary emulsion
9. Primary emulsion formulae- For fixed, mineral, volatile oils and oleo resin
10. Methods of preparation of emulsions
– Trituration method: Dry Gum and Wet Gum
– Bottle method: For volatile oils
Formulation of Emulsions FAQs
1. What are emulsions used for in the food industry? Emulsions are commonly used in the food industry for products like salad dressings, mayonnaise, and ice cream to provide texture and consistency.
2. How do emulsifying agents work? Emulsifying agents have both hydrophilic and hydrophobic parts, allowing them to bridge the gap between oil and water, reducing surface tension and preventing separation.
3. Are natural or synthetic emulsifiers better? The choice between natural and synthetic emulsifiers depends on the specific application. Both have their advantages, and the selection should align with the desired properties and regulations for the product.
4. Why is temperature control important in emulsion preparation? Temperature control is crucial as some emulsions require specific temperature ranges to maintain stability. Deviating from the recommended temperature can result in separation or undesirable texture.
5. What is the significance of the HLB value for emulsifying agents? The Hydrophilic-Lipophilic Balance (HLB) value indicates the emulsifying agent’s compatibility with either water or oil. It helps in choosing the right emulsifier for a particular formulation.
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf