Poison and Antidote

Poison and Antidote
Learning Objectives
At the end of this lecture, the student will be able to:
• Describe antidote
• Cyanide poisoning
• Explain the monograph analysis of: Sodium nitrite, Sodium thio sulphate, Activated charcoal
Poison
A poison is a substance that, when ingested, inhaled, or otherwise absorbed into the body, can cause harm or be fatal. Poisons can come from various sources, including chemicals, toxins, plants, or certain foods. They can have a wide range of effects on the human body, from mild discomfort to severe illness.
Antidote:
It may be defined as those substances, which react specifically with an ingested poison or toxic substance or an overdosage of a potent drug. They either neutralise the poison or its toxic effect or pharmacologically or chemically by converting them to non-toxic or less toxic forms.
An antidote is an agent which counteracts:
• A poison
• Overdosage of a potent drug
• Toxic substance
Mechanism of Action of Antidotes
Antidotes act by different mechanisms. The mechanisms of action of antidotes is given below:
1) Complex formation
2) Metabolic conversion
3) Prevention of toxic metabolite formation
4) By changing the physio-chemical nature of toxicant
5) Promotes return to normal function by repairing a defect or enhancing a function that corrects the effects of poison
Classification of Antidotes
Depending on their action, antidotes are classified as:
1) Chemical Antidotes: They act by usually by combining with the poison and thus changing the chemical nature of the poison so that it becomes inactive.
Eg: Sodium thio sulphate converts systemically toxic cyanide to nontoxic thio cyanate.
2) Physiological Antidotes: They act by producing the effect opposite to that of poison, or counter act the effect of poison physiologically.
Eg: Sodium nitrite converts hemoglobin to meth hemoglobin and prevents binding of cyanide ion.
3) Mechanical Antidotes: They act by usually preventing the absorption of poison in the body or expel out the poison by emesis or elimination through urine.
Eg: activated charcoal absorbs the toxic or poisons before it gets absorb across the intestinal wall.
Cause of Poisoning
Poisoning of the body can be ascertained due to various reasons:
The most common poisoning occurs due to environmental contamination with heavy metals. This has led to food and water contamination.
The poisoning also occurs because of insecticides or pesticides.
The poisoning can also occur due to excessive use of drugs.
The poisoning can also be due to intentional also to commit suicide.
Antidotes are generally discussed on heavy metals and cyanide poisoning.
Different types of poisoning
• Cyanide poisoning
• Heavy metal poisoning
• Food poisoning
• Water poisoning
Cyanide poisoning
Cyanide poisoning is a life-threatening condition caused by exposure to cyanide, a highly toxic chemical. It can result from industrial accidents, smoke inhalation, or the consumption of certain foods. Symptoms include rapid breathing, confusion, headache, and, in severe cases, seizures and loss of consciousness. Immediate medical attention is crucial in cases of suspected cyanide poisoning to prevent severe health consequences.
Cyanide Poisoning Causes
Cyanide poisoning is a severe medical condition caused by exposure to cyanide, a highly toxic chemical compound. Common sources of cyanide exposure include industrial accidents, smoke inhalation, and the consumption of certain foods like apricot kernels.
Cyanide Poisoning Symptoms
The symptoms of cyanide poisoning can range from rapid breathing, confusion, and headache to more severe manifestations such as seizures and loss of consciousness. Prompt medical attention is essential in cases of suspected cyanide exposure, as delay can lead to life-threatening consequences.
Mechanism of action of Cyanide
• Inhibits cellular respiration – Cytochrome oxidase
• Tissues cannot utilize oxygen
• Tissues die
• Finally the person dies
Mechanism of action of drugs used for cyanide poisoning
Clinical Effects of Cyanide posioning
• CNS
– Headache
– Dizziness
– Seizures
– Coma
• Cardiovascular
– Hypertension, bradycardia
– Hypotension, later in course
– Cardiovascular collapse
Treatment for cyanide poisoning
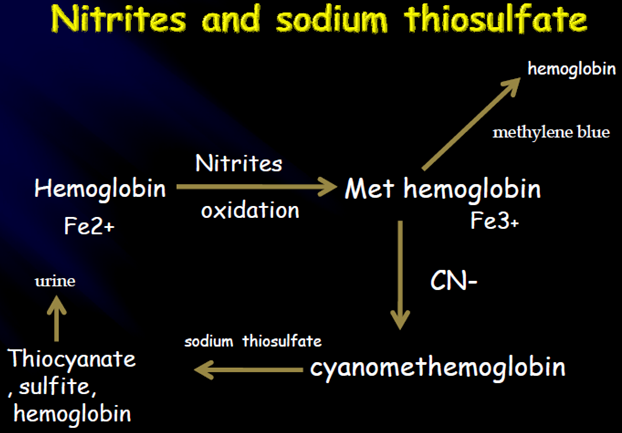
The inorganic compound used are Sodium nitrite followed by Sodium thio sulphate
For cyanide poisoning two inorganic compounds, sodium nitrite and sodium thio sulphate is administered intravenously. The action of these two compound as follows:
Sodium nitrite is able to convert haemoglobin to meth haemoglobin, thus cyanide poisoning of Cytochrome enzyme is prevented. This process is reversible. So immediately after the injection of sodium nitrite a slow intravenous infusion of sodium nitrite is given. The nitrite ion reacts with the cyanide ion and converts to nontoxic thiocyanate, which is eliminated out through urine.
Monograph of Sodium nitrite
Name: Sodium nitrite
Chemical formula: NaNO2
Molecular weight: 68.09
Standards: Sodium nitrite contains not less than 97 per cent and not more than 101.0 per cent of NaNO2
Method of Preparation:
2Na2CO3 + 4NO + O2 à 4NaNO2 + 2CO2
Medical uses:
• Antidote for cyanide poisoning
• Vasodilator
Monograph of Sodium thiosulphate
Name: Sodium thiosulphate
Chemical formula: Na2S2O3,5H2O
Molecular weight: 248.2
Standards: Sodium Thiosulphate contains not less than 99.0 percent and not more than 101.0 per cent of Na2S2O3,5H2O
Synonym: sodium hyposulphate or “hypo” or antichlor
Method of preparation:
2Na2CO3 + H2O + 2SO2 à NaHSO3 + CO2
NaHSO3+ Na2CO3 à 2Na2SO3 + H2O + CO2
Na2SO3 + S à Na2S2O3
6 NaOH + 4S à 2Na2S + Na2S2O3 + 3H2O
Properties of sodium thiosulphate:
Description:
• Colourless large crystals or a coarse, crystalline powder
• Odourless
• Deliquescent in moist air and effloresces in dry air at temperature above 33º C
• It dissolves in its water of crystallisation at about 49ºC
Test for purity:
Appearance of solution
pH
Arsenic
Heavy metals
Chlorides
Sulphides
Sulphates and sulphites
Monograph of Sodium thiosulphate cont…
Assay: principle
Iodimetric titration
2Na2S2O3 + I2 à 2NaI + Na2S4O6
Indicator: starch
Colour change: colourless to pale blue
Storage: Store protected from moisture
Medicinal uses:
• Anti-dote for cyanide poisoning
• Parasitic skin diseases
Monograph of Activated charcoal
Name: Activated charcoal
Activated Charcoal is obtained from vegetable matter by suitable carbonisation processes intended to confer a high adsorbing power
Synonyms: Universal antidote, Decolorising Charcoal
Method of Preparation:
It is prepared from natural vegetable
It involves the following steps:
Step 1. Wood, coconut shell heated at 6000C in absence of air à Vegetable Charcoal
Step 2. Vegetable Charcoal heated at 5000C to9000C in Exposed to air/ steam/ carbon dioxide/sulphuric acid/Phosphoric acid/ zinc chloride à Activated charcoal
• Storing in a suitable container
Test for purity
• Copper
• Chlorides
• Zinc
• Acid-soluble substances
• Acidity or alkalinity
• Lead
• Loss on drying
• Ethanol-soluble substances
• Absorbing power
• Uncarbonised constituents
• Sulphated ash
• Sulphide
• Alkali-soluble coloured matter
• Sulphates
Storage: Store protected from moisture
Medicinal uses:
• Adsorbent in food and alkaloidal poisoning
• Absorbs various gases and toxins
• Dyes and decolourising agent
Antidotes are generally discussed on heavy metals and cyanide poisoning.
Heavy metals poisoning
The common heavy metals responsible for poisoning are the salts of arsenic, lead, mercury, iron and cadmium. Heavy metals poisoning occur because of over dose intake or because of their incomplete metabolism on the body. The mechanism involved in treating the heavy metal poisoning is by administering the drugs, which form a chelate with poison, and converting them into the non-toxic substance and eliminating out from the body through oral or through urine.
The initial treatment for heavy metal poisoning is administration of activated charcoal for absorbing the heavy metal or poison and followed by drugs, which causes emesis so that the poison is removed from circulation.
Some of the inorganic compounds used as antidotes for heavy metal poisoning are copper sulphate, magnesium sulphate, sodium phosphate, etc.
Besides these there are organic compounds which are widely used. They are:
1. D-Pencillamine: Used for copper, magnesium, and lead poisoning.
2. Deferoxamine: Against iron.
3. Dimercarpol: Arsenic, gold, mercury poisoning.
4. Succimer: Arsenic, lead, mercury poisoning.
5. Calcium disodium EDTA: Universal antidote as it forms complex with most of the heavy metals. It is not much used because of its side effect. The universal antidote is activated charcoal.
Frequently asked questions:
- What are antidotes, and how do they work?
Antidotes are substances or treatments that can counteract the effects of poison, toxins, or harmful substances when they enter the body. They work by either neutralizing the toxic substance or rendering it less harmful. For example, Sodium Nitrite and Sodium Thiosulfate are antidotes for cyanide poisoning as they convert cyanide into less harmful forms.
- What are the common sources of cyanide poisoning?
Cyanide poisoning can occur through various sources, including industrial accidents, smoke inhalation, certain foods (like apricot kernels), and exposure to certain chemicals. It’s important to be aware of these potential sources and take precautions.
- Can I administer antidotes at home in case of poisoning?
Administering antidotes is a medical procedure and should be performed by trained healthcare professionals. In cases of poisoning, it is crucial to seek immediate medical attention rather than attempting to administer antidotes at home.
- What are the potential side effects of antidotes?
The potential side effects of antidotes can vary depending on the specific antidote used. Common side effects may include nausea, vomiting, and in some cases, allergic reactions. It’s essential to follow the guidance of medical professionals when administering antidotes.
- Are antidotes always effective in treating poisoning?
The effectiveness of antidotes depends on various factors, including the type and amount of poison, the timing of administration, and the overall health of the individual. While antidotes can be highly effective, there are no guarantees, and prompt medical attention is always crucial in cases of poisoning.
Summary:
•Antidote: used against toxic substances, poisoning and overdose of potent drug
• Cyanide poisoning treatment: Sodium nitrite and sodium thio sulphate
• Activated Charcoal: Universal antidote, as it has good absorbing property
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf