Diabetes Mellitus
Objective
• At the end of this lecture, students will be able to explain
• Explain the biochemical aspects of DM
• Distinguish type 1 from type 2 DM
• Interpret blood glucose levels in fasting states
Overview
• DM is a heterogeneous group of syndromes characterized by an elevation of fasting blood glucose caused by absolute or relative deficiency of insulin
• Two types of DM:
Type 1 (insulin-dependent DM)
Type 2 (noninsulin-dependent DM)
• Prevalence of type 2 increasing with:
Aging (increase in the rate of life-age of the population)
Increasing prevalence of obesity
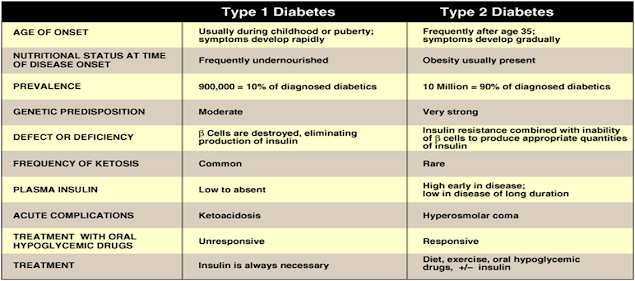
Comparison between type 1 & type 2 DM
Type 1 Diabetes Mellitus
About 10% having DM
• Onset: usually during childhood
• Caused by an absolute deficiency of insulin :
• may be caused by an autoimmune attack of b-cells of the pancreas, viral infection, or toxin
• Destruction is enhanced by environmental factors such as viral infection & a genetic
• Rapid symptoms appear when 80-90% of the b-cells have been destroyed
• Insulin is the only treatment
The onset of type 1 DM
Metabolic changes of type 1 DM
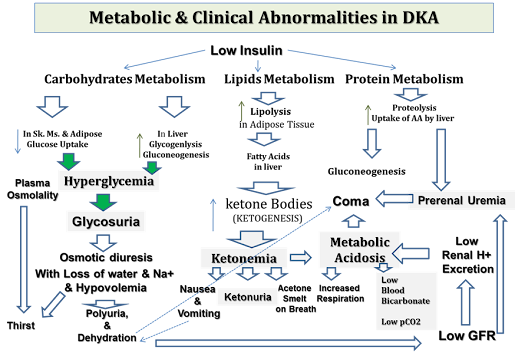
1- Hyperglycemia
2- Diabetic Ketoacidosis (DKA)
3- Hypertriacylglyceridemia & hypercholestrolemia
1- Hyperglycemia:
Increased glucose in the blood
Due to: Decreased glucose uptake by muscles & adipose tissues (by GLUT-4) & Increased hepatic gluconeogenesis (& glycogenolysis)
2- Diabetic Ketoacidosis (DKA):
Increased ketone bodies in the blood (ketonemia) lead to metabolic acidosis
DKA occurs in untreated or uncontrolled cases of DM
– In 25 – 40% of newly diagnosed type 1 DM (untreated & uncontrolled yet)
– In stress states demanding more insulin (as during infection, illness, or during surgery Uncontrolled DM)
– No compliance with therapy (intake of meals with no insulin medication i.e. Uncontrolled DM)
Diagnosis of DKA
1- History (for a cause of DKA)
2- Clinical Examination
3- Lab Investigations: (to confirm the diagnosis & follow up of treatment)
– Urine by dipstick: Glucose & Ketones +++ (RAPID TEST)
– Blood Chemistry Analysis:
* Blood Glucose: High
* Blood Urea: High (due to dehydration)
* Electrolytes:
Low (or normal) sodium
High (or normal) potassium
* Assessment of acid-base status: (metabolic acidosis)
– Blood Bicarbonate: Low (usually below 5 mmol/L)
– pCO2: Low (compensatory)
Biochemical Basis of Treatment of DKA
AIM: (EMERGENCY TREATMENT)
1- Correction of dehydration (Hypovolemia): by IV fluids & Sodium
2- Correction of acidosis: by IV bicarbonate
3- Correction of metabolic abnormality: by insulin IV infusion
4- Potassium is given with insulin treatment as insulin induces K+ entry into cells
5- IV GLUCOSE SHOULD BE STARTED IN CASE of GLUCOSE IN BLOOD FALLS BELOW 10 mmol/L (AVOID HYPOGLYCEMIA INDUCED BY INSULIN)
6- FOLLOW UP is QUITE IMPORTANT to monitor
*Blood glucose level
*Electrolytes (Na+ & K+)
*Acid-base status (blood bicarbonate level)
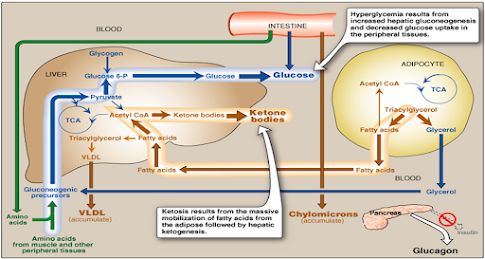
3- Hypertriacylglyceridemia & hypercholesterolemia:
- Released fatty acids from adipose tissues are converted to triacylglycerol & cholesterol in the liver.
Triacylglycerol is secreted from the liver in VLDL to blood (with liver cholesterol)
- Chylomicrons
(from diet fat) accumulates (due to low lipoprotein lipase activity as a result of low or absent insulin)
Chylomicrons contain Triacylglycerols (mainly) & Cholesterol
Increased VLDL & chylomicrons in blood result in hypertriacylglyceridemia & hypercholesterolemia
Diagnosis of type 1 DM
• Clinically:
Age: during childhood or puberty (< 20 years of age)
With Abrupt (Sudden) appearance of Polyuria, Polydipsia, Polyphagia, Fatigue, Weight loss
Complication as ketoacidosis (common, may be the cause of diagnosis)
• Laboratory diagnosis:
Fasting blood glucose: > or equal to 126 mg/dl
100 – 125 mg/dl is called impaired fasting blood glucose
HBA1c: High (more than 6% of normal hemoglobin)
Insulin level in blood: low
Circulating islet-cell antibodies detection
Biochemical Aspects for Treatment & Control of Type 1 DM
AIM
Exogenous insulin by subcutaneous injection is given to:
1- Control Hyperglycemia (long-run complications)
2- Prevent the occurrence of Ketoacidosis (emergency case!!)
Strategies of Treatment
1- Standard Treatment
2- Intensive Treatment (Tight Control)
1- Standard Treatment:
By one or two injections of insulin/per day
AIM: Mean blood glucose level 225-275 mg/dl (normal: 110 mg/dl)
HbA1c level: 8-9 % of total Hb (normal: 6% of total hemoglobin)
HbA1c: HbA1c is proportional to average blood concentration over the previous several months
So, it provides a measure of how proper treatment normalized blood glucose in diabetics over several months
2- Intensive Treatment: (Tight control)
By more frequent monitoring & subsequent injection of insulin (i.e. 3 or more times/day)
It will more closely normalize blood glucose to prevent chronic complications of the existence of hyperglycemia for a long period.
AIM: Mean blood glucose levels of 150 mg/dl
HbA1c: approximately 7% of total hemoglobin
Advantage: Reduction in chances of occurrence of chronic complications of DM: e.g. retinopathy, nephropathy & neuropathy by about 60%
Complications of Treatment by Insulin
Hypoglycemia is a common complication of insulin treatment (in more than 90% of patients on insulin medication)
More Common with intensive treatment strategy
Causes of hypoglycemia due to insulin treatment
Diabetics cannot depend on glucagon or epinephrine to avoid hypoglycemia as:
No glucagon (early in the disease)
No epinephrine (with the progression of the disease diabetic autonomic neuropathy with the inability to secrete epinephrine in response to hypoglycemia)
So, patients with long-standing type 1 DM are particularly vulnerable to hypoglycemia
Contraindications of Intensive Treatment
• Children: risk of episodes of hypoglycemia may affect the brain development
• Elderly people: hypoglycemia can cause strokes & heart attacks in older people
Type 2 Diabetes Mellitus
Type 2 DM
• 90% of diabetics (in the USA)
• Develops gradually
• may be without obvious symptoms
• may be detected by routine screening tests
• BUT: many types 2 diabetics have symptoms of polyuria & polydipsia
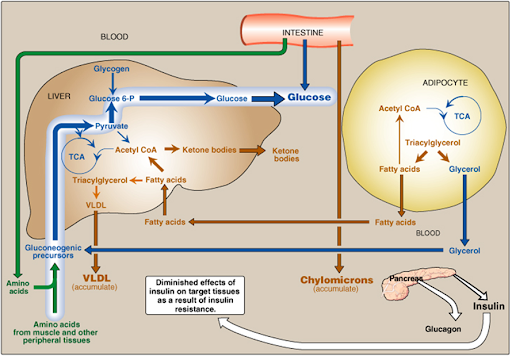
• In type 2 DM: a combination of insulin resistance & dysfunctional b-cells
• Metabolic changes in type 2: are milder than in type 1, as insulin secretion, although not adequate, restrains ketoacidosis
Causes of Type 2 DM
Insulin Resistance & Dysfunctional b-cell
Insulin resistance is the decreased ability of target tissues, such as the liver, adipose tissue & muscle to respond properly to normal circulating insulin
Obesity is the most common cause of insulin resistance
Obesity causes insulin resistance as:
– Substances produced by fat cells as leptin and resistin may contribute to the development of insulin resistance
– Free fatty acids elevated in obesity are involved in insulin resistance
Obesity, Insulin Resistance & DM
- Obesity is the most common cause of insulin resistance.
HOWEVER, Most people with obesity & insulin resistance do not develop DM!!
- How insulin resistance leads to DM??
1- In the absence of a defect in b-cell function, nondiabetic, obese individuals can compensate for insulin resistance by secreting high amounts of insulin from b-cell (i.e. Hyperinsulinemia)
So, glucose levels in the blood remain within the normal range
2- In late cases, b-cell dysfunction with low insulin secretion occurs due to increased amounts of free fatty acids & other factors secreted by fat cells (as leptin & resistin) may end in the development of type 2 DM (hyperglycemia).
In Type 2 DM
Initially (In early stages:
with Insulin resistance) the pancreas retains b-cell capacity
â
Insulin is secreted
(maybe higher than normal i.e. hyperinsulinemia)
â
Normal blood glucose
levels
With time (late stages)
b-cells become dysfunctional (low
function) (due to the harmful effects of FFAs & substances released by increased
fat cells)
â
b-cells fail
to secrete enough insulin (low insulin)
â
Increased blood glucose levels (hyperglycemia)
Metabolic changes in Type 2 DM
Metabolic abnormalities of type 2 DM are the results of insulin resistance (in the liver, muscle & adipose tissue)
1- Hyperglycemia
2- Hypertriacylglyceridemia
3- Nonketotic hyperglycemic coma
In cases with severe hyperglycemia especially in older age diabetics with type 2
Hyperglycemia induces osmotic diuresis with loss of ECF
The osmotic diuresis causes loss of water in excess of sodium leading to very high plasma osmolality (with hypernatremia) & marked dehydration
No ketogenesis due to the presence of sufficient insulin to prevent DKA (or sometimes there is minimal ketogenesis with minimal metabolic acidosis i.e. Bicarbonate is not much lowered as in DKA)
Treatment:
Fluid replacement + Insulin IV infusion + follow-up (Emergency Case!!)
Chronic Effects of DM
The long-standing hyperglycemia causes the chronic complications of DM
1- Atherosclerosis: Diabetic Retinopathy
Diabetic Nephropathy: glomerular proteinuria
Diabetic Neuropathy: peripheral neuritis
Cardiovascular Diseases (as MI) & strokes (as cereb. hge)
2- Sorbitol accumulation in certain cells with its complications
3- Glycated proteins formation with microvascular complications
For avoiding these complications, long-term control of hyperglycemia is recommended for all types of DM
In cells where entry of glucose is not
dependent on insulin (eye lenses,
retina, kidney, neurones)
â
á Intracellular Levels of Glucose
â
á SORBITOL accumulation in these
cells
â
Cataract
Diabetic Retinopathy
Diabetic Nephropathy
Diabetic Neuropathy
Treatment of Type 2 DM
• AIM:
1- To maintain blood glucose concentrations within normal limits
2- To prevent the development of long-term complications occurring due to prolonged hyperglycemia
• Lines of treatment:
1- Weight reduction (to control insulin resistance)
2- Exercise
3- Dietary modification
4- Hypoglycemic agents
5- Insulin (required in some cases)
Case Study
The parents of a 15 years old boy were reported by his school that he was found drowsy & they have got to take him to hospital according to the advice of his school doctor.
In the hospital, his mother told the doctor that her son seemed unusually thirsty for the last 3 months & she thought that he had lost weight. She admitted also that on the morning before leaving for school, he was complaining of abdominal pain & discomfort.
On examination:
- Semiconscious
- Deep & rapid respiration
- Pulse rate 120 beats/minute
- BP: 90/50
- Cold extremities
What investigations were recommended for him??
What is the diagnosis of this case??
What is the treatment??
Clinical Biochemistry Lab Investigations
Blood Chemistry
Random Blood Glucose: 550 mg/dl
Urea: 160 mg/dl (N: 20 -40)
Na+: 127 mmol/L (N: 135 – 145)
K+: 6.9 mmol/L (N: 3.5 – 4.5)
pCO2: 2.9 kPa (N: 4.4 – 6.1)
HCO3- : 7 mmol/L (N: 21 – 27.5)
pO2: 14 kPa (N: 12 – 17)
Urine Analysis:
Urine Dipstick Test:
– Glucose +++
– Ketone +++
– Albumin ++
Summary
• Type 1 is insulin depended
• Type 2 is insulin-independent
• Complications are more dangerous
• Lab abnormalities
– Altered sugar values
– Proteinuria
– HbA1C
Also, Visit: Health and Wellness