Respiratory chain, its role in energy capture and its control

Objective
• At the end of this lecture, student will be able to
– Explain Respiratory chain
– Describe structural organisation of ETC
– Discuss components of ETC
– Discuss Inhibitor of ETC
Respiratory chain / Electron Transport Chain
• The respiratory chain, often referred to as the electron transport chain (ETC), is an indispensable component of cellular respiration.
• The energy rich carbohydrates, fatty acid and amino acid undergo a series of metabolic reaction and are finally oxidized to CO2 and H2O
• The reducing equivalents from various metabolic intermediates are transferred to coenzymes NAD+ and FAD to produce NADH & FADH2
• The latter two reduced coenzymes pass through ETC and finally, reduce oxygen to water
• The passage of electrons through the ETC is associated with the loss of free energy
• A part of this free energy is utilized to generate ATP from ADP and Pi
Mitochondria: The power houses of cell
• Mitochondria are the centres for metabolic oxidative reactions to generate reduced co-enzymes (NADH & FADH2) which are utilized in ETC to liberate energy in the form of ATP. Hence, regarded as the power house of the cell
• It consists of 5 distinct parts, outer membrane, inner membrane, inter-membrane space, cristae and matrix
• ETC & ATP synthesizing system are located on the inner mitochondrial membrane, which is a specialized structure, rich in proteins. It is impermeable to ions (H+, K+, Na+) and small molecules (ADP, ATP)
• This membrane is highly folded to form cristae – increases the inner surface area
• The inner surface consist of phosphorylating subunits which are the centres for ATP production
• Matrix is rich in enzymes responsible for the citric acid cycle, β-oxidation of fatty acids and oxidation of amino acids
Structural organization of respiratory chain
• The inner mitochondrial membrane consist of five distinct respiratory or enzyme complexes, denoted as complex I Il, III, IV and V
• The complexes l-lV are carriers of electrons while complex V is responsible for ATP synthesis
• NADH, coenzyme Q, cytochrome C and oxygen are mobile electron carriers in the respiratory chain
• The enzyme complexes (I-IV) and the mobile carriers are collectively involved in the transport of electrons which, ultimately, combine with oxygen to produce water
• The largest proportion of the oxygen supplied to the body is utilized by the mitochondria for the operation of electron transport chain
Components and reactions of Electron Transport Chain (ETC)
• Five distinct carriers in ETC
• These carriers are sequentially arranged and are responsible for the transfer of electrons from a given substrate to ultimately combine with proton and oxygen to form water
l. Nicotinamide nucteotides:
• Two coenzymes NAD+ & NADP+ derived from the vitamin niacin, NAD+ is more actively involved in the ETC
• NAD+ is reduced to NADH + H+ by dehydrogenases with the removal of two hydrogen atoms from the substrate (AH2)
e.g. glyceraldehyde-3-phosphate, pyruvate, isocitrate, α-ketoglutarate and maleate
• NADPH + H+ produced by NADP+dependent dehydrogenase is not used in a substrate for ETC. NADPH is more effectively utilized for anabolic reactions (e.g. fatty acid synthesis, cholesterol synthesis)
2. Flavoproteins:
• The enzyme NADH dehydrogenase is a flavoprotein with FMN as the prosthetic group. The coenzyme FMN accepts two electrons and form FMNH2
• NADH dehydrogenase is a complex enzyme closely associated with non-heme iron proteins (NHI) or iron-sulfur proteins (FeS)
• Succinate dehydrogenases is an enzyme found in the inner mitochondrial membrane. lt is also a flavoprotein with FAD as the coenzyme. It can accept two hydrogen atoms from succinate
3. Iron sulfur (FeS) proteins:
• FeS proteins exist in the oxidized (Fe3+) or reduced (Fe2+) state
• One FeS participates in the transfer of electrons from FMN to coenzyme Q
• Other FeS proteins associated with cytochrome b and cytochrome c1 participate in the transport of electrons
4. Coenzyme Q (ubiquinone):
• lt is a quinone derivative with a variable isoprenoid side chain
• The mammalian tissues possess a quinone with 10 isoprenoid units which is known as coenzyme Q10
• Coenzyme Q is a lipophilic electron carrier- lt accepts electrons from FMNH2 produced in the ETC by NADH dehydrogenase
5. Cytochromes:
• The cytochromes are conjugated proteins containing heme group, consists of a porphyrin ring with iron atom
• The iron of heme in cytochromes is alternately oxidized (Fe3+) & reduced (Fe2+), which is essential for the transport of electrons in the ETC
• Three cytochromes were initially discovered from the mammalian mitochondria- designated as cytochrome a, b & c depending on the type of heme present and the respective absorption spectrum
• Additional cytochromes such as c1, b1, b2, a3 etc were discovered later
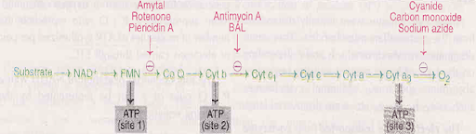
Inhibitors of Electron Transport Chain (ETC)
• The inhibitors bind to one of the components of ETC and block the transport of electrons & causes the accumulation of reduced components
• The synthesis of ATP is dependent on electron transport. Hence, all the site-specific inhibitors of ETC also inhibit ATP formation
• 3 possible sites
1. NADH and coenzyme Q : Fish poison, rotenone, barbiturate drug amytal and antibiotic piercidin A inhibit this site
2. Between cytochrome b and c1: Antimycin A – an antibiotic, British antilewisite (BAL) –an antidote used against war-gas-are the two important inhibitors of the site between cytochrome b and c1
3. Inhibitors of cytochrome oxidase: Carbon monoxide, cyanide, hydrogen sulphide and azide effectively inhibit cytochrome oxidase
Summary
• The energy rich carbohydrates, fatty acid and amino acid undergo a series of metabolic reaction and finally oxidized to co2 and H2O
• The passage of electrons through the ETC is associated with the loss of free energy and part of this free energy is utilized to generate ATP from ADP and Pi
• Mitochondria are the centres for ETC
• The components of ETC are nicotinamide, Flavoproteins, Iron sulfur proteins, Coenzyme Q
• Fish poison, rotenone, barbituate drug amytal, piercidin A, Antimycin A, British antilewisite (BAL), Carbon monoxide, cyanide, hydrogen sulphide and azide are inhibitors of ETC
FAQs
1. Why is the respiratory chain essential for our cells?
The respiratory chain is vital because it generates ATP, the primary energy source for cellular activities.
2. How do inhibitors affect the electron transport chain?
Inhibitors disrupt the normal flow of electrons, leading to a decrease in ATP production and potentially impacting cell function.
3. What happens if the electron transport chain is inhibited?
Inhibition of the ETC can lead to a decrease in energy production and can have various physiological consequences.
4. Are inhibitors of the ETC used in medical research?
Yes, ETC inhibitors are used in research to study cellular respiration and develop therapeutic interventions.
5. What are the potential dangers of ETC inhibitors like cyanide and carbon monoxide?
Cyanide and carbon monoxide are highly toxic and can be lethal when they inhibit the respiratory chain, leading to severe health consequences.
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf