Sedatives and Hypnotics
Intended learning outcomes
At the end of this lecture, student will be able to:
• Define and differentiate between Sedatives and hypnotics
• Categorize the sedative-hypnotic agents
• Describe the SAR of Barbiturates
• Outline the synthesis of Barbiturates
• Discuss the SAR of Benzodiazepines
• Outline the synthesis of some benzodiazepines
• Discuss about some miscellaneous sedative-hypnotic drugs
CNS Depressant
• CNS Depression refers to physiological depression of the central nervous system that can result in decreased rate of breathing, decreased heart rate, and loss of consciousness possibly leading to coma or death.
Definition and differentiation
• Sedative: A drug that reduces excitement, calms the patient (without inducing sleep)
• Sedatives in therapeutic doses are anxiolytic agents
• Most sedatives in larger doses produce hypnosis (Trans
like state in which subject becomes passive and highly suggestible)
• Site of action is on the limbic system which regulates
thought and mental function
• Hypnotic: A drug which produces sleep resembling natural sleep
• They are used for initiation and / or maintenance of
sleep.
• Hypnotics in higher doses produce General anaesthesia.
• Site of action is on the midbrain and ascending RAS which
maintain wakefulness.
Stages of Sleep
History of Sedatives and hypnotics
• Sedatives (before development of barbiturates)
– Since antiquity, alcohol beverages and potions containing
laudanum and various herbals have been used to induce sleep.
– Morphine was used for quick management of aggressive patients
In 1800: most widely used sedative in asylums
– In 1857: Bromide was the first agent to be introduced
specifically as a sedative and soon thereafter as a hypnotic
• 1864: Barbiturates were introduced
– Barbiturates were widely diverted from medical use and
used on the street in the 60s where they were called “downers” and sold under a variety of different names.
– Barbiturates had a low therapeutic index and were often
used for suicide.
Baeyer, discoverer of barbiturates
Marilyn Monroe died of barbiturate overdose in 1962
• By 1990s, barbiturates replaced by benzodiazepines
– 1961: introduction of chlordiazepoxide
– Sternback is credited with the invention of chlordiazepoxide, diazepam, flurazepam, nitrazepam, clonazepam, and trimethaphan
Leo Sternback
• Others and Z Drugs
– Methaqualone (Quaalude) and meprobamate (Miltown) were used in the 60s as “non barbiturate tranquilizers”.
• 1951: Methaqualone was synthesized in India as an
antimalarial
• 1965: the most commonly prescribed sedative in Britain
• 1972: the sixth-bestselling sedative in the USA
• discontinued in 1985, mainly due to its psychological
addictiveness and recreational use
– Z drugs: now replacing the BDZs; can be targeted to
specific symptoms, insomnia and anxiety.
DOSE DEPENDENT ACTION
SEDATION > SLEEP > ANESTHESIA > COMA > DEATH
SEDATIVE- HYPNOTICS CLASSIFICATION
SEDATIVE- HYPNOTICS
1. Barbiturates
2. Non Barbiturates
1. Barbiturates:
They are further classified as follows:
a. Long-acting barbiturates (6 h or more than 6 h)
Example: barbital, phenobarbital, Mephobarbital, Metharbital
b. Intermediate-acting barbiturates (3–6 h)
Example: Amobarbital, Butabarbital, Aprobarbital, Talbutal, Butalbital, Hexobarbital
c. Short-acting barbiturates (less than 3 h)
Example: Pentobarbital, Secobarbital, Cyclobarbital,
Heptabarbital
d. Ultra short-acting barbiturates (15 min)
Example: Thiopentone
2. Non-barbiturates:
They are further classified as follows;
Benzodizepine derivatives: Chlordiazepoxide, diazepam, oxazepam, alprozolam, flurazepam, triazolam, prazepam, halazepam, temazepam, lorazepam
Miscellaneous
(a) Aldehydes and their derivatives: Chloral hydrate, paraldehyde, triclofos sodium
(b) Piperidine derivatives: Glutethimide, methyprylone
(c) Quinazoline derivatives: Methaqualone
(d)Alcohols and their carbamate derivatives: Ethchlorvynol, meprobamate, ethinamate
Mechanism of Action of Sedatives
Barbiturates / Benzodiazepines
At higher dose
â
Bind to GABAA
receptor at different allosteric sites
â
Facilitates GABA
action (Barbiturates can act as GABA mimetic)
â
Barbiturates increase
duration & Benzodiazepines increase frequency of opening of Cl- channel
â
Membrane
hyperpolarization
â
CNS depression
Chemistry of Barbiturates
1. Barbiturates are derivatives of barbituric acid (2,4,6-trioxyhexahydropyrimidine) which is devoid of hypnotic and sedative activities
2. Barbituric acid may be described as a “cyclic ureide of
malonic acid”. Barbituric acid can be made by condensing urea with ethyl malonate in presence of sodium ethoxide.
3. Clinically important hypnotic-sedative barbiturates have
substitutions at sites 1, 2 and, especially, 5 of barbituric acid.
4. Keto-enol tautomerism of barbituric acid and barbiturates
allows formation of water soluble salts with a strong base
5. The barbiturates do not dissolve readily in water, their
sodium salts dissolve readily in water.
6. Buffering action of Na2CO3 plus atmospheric CO2 maintains pH at 10 to 11. In less alkaline solutions, these barbiturates may precipitate as the free acids; so
do not reconstitute barbiturates with normal saline and do not mix with acidic solutions of other drugs
Structure-Activity Relationships (SAR) of Barbiturates
1. Hypnotic activity.
Side chains at position 5 (especially if one of them is branched) is essential for activity
• Both the hydrogen atoms at 5th position should be
replaced.
This may be because if one of the hydrogen atom is available at 5th position keto-enol tautomerism is possible.
• If lower alkyl groups are attached to the 5th position
there is an increase in the onset of action and duration of action
• Lower alkyl group causes lipophilicity and an ability to
cross BBB
2. Potency and duration of action. Length of side chain at position 5 influences potency and duration of action.
– Ex: Secobarbital and thiamylal are slightly more potent
than pentobarbital and thiopental, respectively.
3. More rapid onset and shorter duration of action. Sulfur instead of oxygen atom at position 2 has more rapid onset of action but shorter duration.
– Ex: thiamylal and thiopental have more rapid onset and
shorter duration of action than secobarbital and pentobarbital,
4. Increased incidence of excitatory side effects. Methylation at position 1 (methohexital) enhances excitatory side effects.
5. Increased potency, rate of onset and short action.
Generally an increase in the lipophilicity of the compound results in more rapid onset of action accompanied with an increase in potency.
6. Introduction of polar groups (hydroxyl, keto, amino, or
carboxyl) into C-5-alkyl sidechain makes the compound more hydrophilic in nature.
– Due to the polar nature, hydrophilic barbiturates do not
dissolve in microsomal membranes of liver and are excreted.
7. Branched, cyclic or unsaturated side chain at C-5
position generally reduce the duration of action
– Due to an increased ease of metabolic conversion to a more polar, inactive metabolite.
8. Stereoisomerism.
Though their l-isomers are nearly twice as potent as their d-isomers, barbiturates are marketed as racemic mixtures.
– Methohexital has two asymmetric carbon atoms, so exists as 4 stereoisomers
• N methylation decreases duration of action by increasing the concentration of lipid soluble free barbituric acid-good absorption in GIT, and substantial binding to protiens
• Group substituted at 5th position like phenyl, alkyl and
presence of double bond, triple bond can influence the ease of oxidative metabolism.
• Thiobarbiturates have a very low duration of action because of the lipid water partition coefficient is high.
Uses of Barbiturates
1. Barbiturates may be used before surgery to relieve anxiety or tension.
2. In addition, some of the barbiturates are used as
anticonvulsants to control seizures in certain disorders or diseases, such as epilepsy.
3. The barbiturates have been used to treat insomnia
(trouble in sleeping); but if they are used regularly (for example, every day) for insomnia, they are usually not effective for longer than 2 weeks.
4. The barbiturates have also been used to relieve
nervousness or restlessness during the daytime. However, the barbiturates have generally been replaced by safer medicines for the treatment of insomnia and daytime nervousness or tension.
If too much of a barbiturate is used, it may become
habit-forming.
Barbiturates should not be used for anxiety or tension
caused by the stress of everyday life.
These medicines are available in the following dosage forms: capsules, tablets, elixir, injection, suppositories
Barbitone sodium (Barbital sodium)
• Baritone sodium exists as white, crystalline powder or
colourless crystals that is soluble in boiling water and in alcohol, but only slightly soluble in water. It forms water-soluble salts with sodium hydroxide.
Medicinal Uses
• Sedative hypnotic
• Used in the treatment of epileptic seizures
BARBITONE SYNTHESIS
Barbitone is prepared by the condensation of urea with
diethyl malonic ester in the presence of sodium ethoxide with the elimination of two molecules of ethanol
PHENOBARBITAL INN, USAN, PHENOBARBITONE BAN
• Phenobarbitone sodium is hygroscopic, bitter taste, water
soluble, odorless, white crystalline powder.
Medicinal Uses
• It is used both as sedative and hypnotic.
• It is the drug of choice in the treatment of grandma and
petitmal epilepsy
• It is useful in nervous and related tension states.
• An overdose of it can result in coma, severe respiratory
depression, hypotension leading to cardiovascular collapse, and renal failure.
METHYL PHENOBARBITONE (MEPHOBARBITAL)
Mephobarbital is white crystalline, water insoluble
powder. It is soluble in aqueous solutions of alkali hydroxides and carbonates
Medicinal Uses
• It possesses hypnotic action
• It is used for the relief of anxiety, tension, and apprehension, and is an antiepileptic in the management of generalized tonic-clonic and absence seizures
AMOBARBITAL INN, USAN, AMYLOBARBITONE BAN
• It occurs as white crystalline powder. It is slightly soluble in water but freely soluble in alkali hydroxide and carbonate solutions.
Medicinal Uses
• Sedative hypnotic
• If Amobarbital is taken for extended periods of time,
physical and physiological dependence can develop.
BUTOBARBITAL/BUTABARBITONE (NEONAL)
• It is white crystalline powder, slightly soluble in water.
• Butabarbital is also used in combination with belladonna. The belladonna is added for antispasmodic effect.
Medicinal Uses
• Sedative and hypnotic, especially used for the short-term
treatment of insomnia. Because of tolerance, barbiturates lose efficacy after two weeks of use
• Used in relieving anxiety before surgical procedures
• However it is also relatively dangerous particularly when
combined with alcohol, and so is now rarely used
Pentobarbital Sodium USAN, Pentobarbitone Sodium BAN
• Pentobarbitone and its sodium salt is available as white,
crystalline powder.Pentobarbitone is slightly soluble in water, whereas its sodium salt is freely soluble in water
Medicinal Uses:
• As a veterinary anaesthetic agent
• The treatment of insomnia, Sedative, hypnotic for short
term,as a basal anaesthetic and also in strychnine poisoning
Quinalbarbitone Sodium BAN, Secobarbital Sodium
• It is a white, hygroscopic powder having a bitter taste,
with pH between 9.7 and 10.5, soluble in water and alcohol.
Medicinal Uses
• It is used in status epilepticus and in toxic reactions to strychnine.
• Used for treating sleep disorders.
• Also used as a sedative prior to anesthesia for surgery
• It also has local anaesthetic, anticonvulsant and anxiolytic properties
Benzodiazepine
Introduction
• Benzodiazepine is a group of two-ring heterocyclic compounds consisting of a benzene ring fused to a diazepine ring
• Chemical Names: Benzodiazepine; 1,4-Benzodiazepine
• Molecular Formula: C9H8N2
Common side effects of benzodiazepines:
• Mental slowing, sedation, blurred speech, blurred vision,
anorexia, nausea, vomiting, dry mouth, diarrhoea, and constipation.
Benzodiazepine
• Benzodiazepines are the most commonly used anxiolytics and hypnotics.
• They act at benzodiazepine receptors, which are associated with gamma-aminobutyric acid (GABA) receptors.
• Clinically useful benzodiazepines to treat anxiety are:
– Chlordiazepoxide,
– Diazepam*
– Oxazepam
– Chlorazepate
– Lorazepam
– Alprazolam
– Zolpidem
SAR of Benzodiazepines
• General structure of Benzodiazepines
7th position
– An electronegative substituent at the 7th position is required for optimal activity.
– Electronegativity increases => activity increases
– If this electronegative substituent is present at the 6th,
8th, 9th positions => activity decreases.
5th position
• Phenyl group at the 5th position promotes the
activity.
– Further substitution in the phenyl group with an
electronegative substituent at the 2nd or the 6th positions => enhances activity.
– If this substituent is at the para position =>activity
decreases.
DOUBLE BOND AT 4th -5th POSITIONS
– Saturation of the double bond at 4th-5th position
=>activity decreases
– If the double bond is shifted from the 4th-5th position to
the 3rd- 4th position => activity decreases
3rd POSITION
– If there is an alkyl group at the 3rd position
=>activity decreases
• –OH group at the 3rd position:
– Presence of –OH=>conjugation reaction=>rapid metabolism=>short half-life=>short duration of action.
– Absence of –OH group=>half-life is more=>less
metabolism=>longer duration of action.
2nd POSITION
• Presence of carbonyl group at the 2nd position is required
for optimal activity.
1st POSITION
• The substitution at the 1st position should be small example-methyl group for optimal activity. Example- Diazepam
• FUSION WITH TRIAZOLO OR IMIDAZOLO RINGS
• If a nucleus like triazolo or imidazolo rings are fused at
the 1st and 2nd positions of the diazepine ring => activity enhanced
• Such compounds will get metabolized by hydroxylation of the methyl group of the triazolo or the imidazolo rings =>converted into active hydrogen compounds=>readily conjugated.
– Example Alprazolam
• Triazolobenzodiazepines
• Alprazolam is used to treat anxiety and panic disorders. It works by enhancing the effects of GABA
Summary of SAR of Benzodiazepines
Chlordiazepoxide
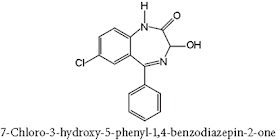
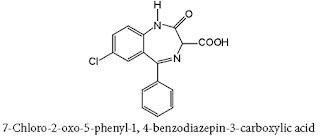
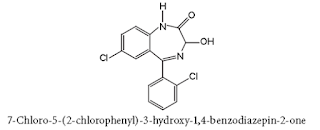
• Chlordiazepoxide hydrochloride is 7-chloro-2-(methylamino) -5- phenyl-3H-1,4-benzodiazepine 4-oxide hydrochloride.
Properties:
• A white to practically white crystalline substance, it is
soluble in water. It is unstable in solution and the powder must be protected from light.
Medicinal Uses:
• Antianxiety: used to relieve fear and anxiety before
surgery
• Sedative
• Appetite-stimulating
• Weak analgesic actions
• Acute alcohol withdrawal
Diazepam (Calmpose, Valium, Diazep)
• Diazepam is a benzodiazepine derivative. Chemically it is 7-chloro-1, 3-dihydro-1-methyl-5-phenyl-2H-1,4-benzodiazepin-2-one
Properties:
• A white or almost white, crystalline powder, very slightly
soluble in water, soluble in alcohol.
Medicinal Uses:
• Diazepam is the drug of first choice for the treatment of
status epilepticus (a particular type of convulsive disorder) when it is given intravenously.
• Used as a skeletal muscle relaxant
• Anticonvulsant and
• Antianxiety agent
DIAZEPAM SYNTHESIS
Oxazepam (Serepax)
Properties and uses:
• It is a white or almost white crystalline powder slightly
soluble in ethanol and insoluble in water.
Medicinal Uses:
• It is useful for the control of acute tremulousness,
inebriation, or anxiety associated with alcohol withdrawal
Chlorazepate
• It is used as a sedative and hypnotic
• It is used to treat anxiety disorders, partial seizures,
or alcohol withdrawal symptoms
• Clorazepate is a water-soluble benzodiazepine derivative
LORAZEPAM
Properties:
• It is a white or almost white crystalline powder, which is
insoluble in water, sparingly soluble in ethanol, sparingly s chloride. It shows polymorphism.
Medicinal Uses:
• It is used as sedative and hypnotic
• It is commonly prescribed for anxiety disorders
• For Insomnia, for alcohol withdrawal, to prevent nausea
& vomiting due to chemotherapy
Alprazolam
Properties:
• It is a white crystalline powder, practically insoluble in
water, freely soluble in methylene chloride sparingly soluble in acetone and in alcohol. It shows polymorphism.
Medicinal Uses:
• Management of insomnia
• Anxiolytic in doses of milligram
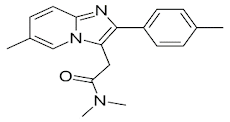
Zolpidem
• Zolpidem is a nonbenzodiazepine and hypnotic of the
imidazopyridine class
• Used in the treatment of insomnia
Miscellaneous sedative-hypnotic drugs
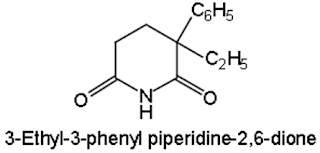
Amides & imides: Glutethmide:
Belongs to Cyclic Hypnotics Containing Nitrogen classification/Piperidinedione derivatives
Properties:
• Gultethemide is colorless or white colored, water
insoluble powder.
• It should be stored in light protected containers
Medicinal Uses:
• Hypnotic in all types of insomnia
• It induces sleep without depressing respiration
Alcohol & their carbamate derivatives: Meprobomate, Ethchlorvynol.
Meprobomate:
• Meprobamate is propanediol derivative
• Chemically it is 2-methyl-2-propyl trimethylene
dicarbamate
Properties:
• Meprobamate is an odorless, white colored crystalline
aggregate with bitter taste
• It is insoluble in water but soluble in alcohol and
slightly soluble in ether
Medicinal Uses:
• Meprobamate is used to induce sleep in anxiety and tensive patients
• It also possesses anticonvulsant and muscle relaxant
properties
Ethchlorvynol:
Properties:
• It is a colourless or slightly yellow transparent liquid.
It solidifies on cooling to form a crystalline mass.
• Miscible with alcohol and with essential oils, soluble in
water, but less soluble in boiling water
Medicinal uses:
• it is exclusively used in the management of hospitalized
patients undergoing alcohol withdrawal
• No longer prescribed- safer drugs available
Aldehyde & their derivatives: Triclofos sodium, Paraldehyde
Triclofos sodium:
• Chemically it is 2,2,2-trichloroethylhydrogen
orthophosphate, which occurs as its sodium salt.
Properties:
• Triclofos sodium is hygroscopic, white colored, water
soluble powder.
Medicinal Uses:
Triclofos is used as hypnotic and sedative
Paraldehyde:
Paraldehyde is a cyclic trimer of acetaldehyde. Chemically
paraldehyde is 2,4,6-trimethyl-1, 3, 5-trioxane.
Properties:
• It is a colorless liquid having strong characteristic odor
• It solidifies on cooling to form a crystalline mass
• Miscible with alcohol and with essential oils, soluble in
water, but less soluble in boiling water
Medicinal Uses: used in the management of hospitalized patients undergoing alcohol withdrawal
Summary
• Sedative are drugs which exert a quietening effect accompanied by relaxation and rest but do not necessarily induce sleep.
• Hypnotics are the drugs which induce sleep by depressing CNS particularly reticular activity.
• Ideally they should cause absolutely little abuse, dependence, addiction or tolerance.
• The general synthesis of barbiturates involves
condensation of the substituted intermediate with urea.
SAR of Barbiturates
• Pyrimidine 2,4,6-trione is essential for activity
• Both the Hydrogens in 5th position should be substituted for activity to avoid keto-enol tautomerism
• Duration of action will depend on the substituents at the 5th position
SAR of Benzodiazepine
• Benzodiazepine ring is essential for activity
– Benzene ring at 5th position is essential for activity
• Triazolobenzodiazepines have antianxiety activity rather
than antidepressant activity
• Synthesis of some benzodiazepines and miscellaneous drugs have been outlined
• Some of the miscellaneous drugs are not used anymore because of the side effects
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf