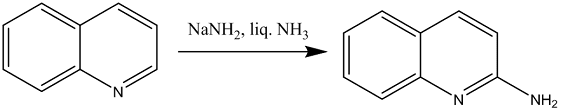Chemistry of Quinoline &
Isoquinoline
Session Objectives
By the end of this
session, students will be able to:
• Discuss various methods of synthesis of Quinoline and
Isoquinoline
• Discuss the chemistry, reactivity, properties of Quinoline
and Isoquinoline
Chemistry
of Quinoline
• Quinoline structure obtained by ortho-condensation of
benzene ring with pyridine
• Also called as azanaphthalene or benzopyridine
• First isolated by Runge in 1834, from coal tar bases
• In 1842 by Gerhardt from the alkaline pyrolysis of
cinchonine, an alkaloid related to quinoline
• Numbering starts from nitrogen atom, which is assigned
postion-1
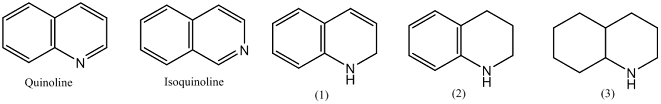
• Dehydrogenation of quinolone yields 1,2-dihydroquinoline
(1), 1,2,3,4-tetrahydroquinoline (2) and decahydroquinoline (3)
Physical properties of Quinoline:
• Quinoline is a colorless hygroscopic liquid with boiling
point 2370C
• Has a characteristic smell resembling that of pyridine
• On exposure to air it develops a yellow color
• Miscible with organic solvents and soluble in water to
extent of 0.7%
• Highly aromatic
• Weakly basic with pKa of 4.94
• Presence of electron-donating groups at 2nd and
4th position increase the basicity, 2-methylquinoline pKa 5.83
Synthetic methods of Quinoline:
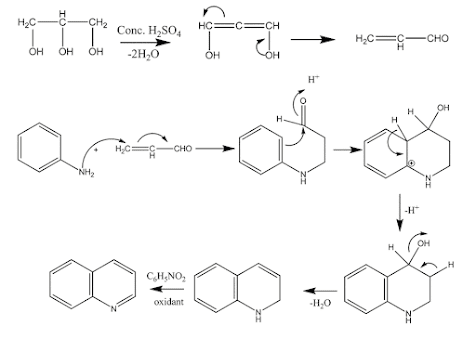
1) The Skraup
synthesis:
• Most important method of preparation
• Involves an aniline derivative is heated with glycerol,
conc. Sulfuric acid and an oxidizing agent (nitrobenzene).
2) The
Friedlander synthesis:
• Most useful method
• o-aminobenzaldehyde or o-aminoacetophenone is condensed
with aldehyde or ketone when refluxed in
presence of alcoholic sodium hydroxide solution to quinoline
• First step is the formation of Schiffs base followed by
ring closure to quinoline by a Knoevenagel condensation.
Chemical properties of Quinoline:
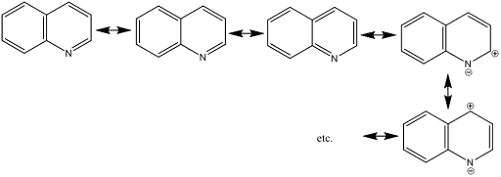
• Because of fusion of benzene ring, properties of both
benzenoid and pyridinoid compounds frequently manifest themselves
1) Reaction
with acids: quinoline is a weak base and gets protonated on ring nitrogen
with mineral acids to form water soluble salts
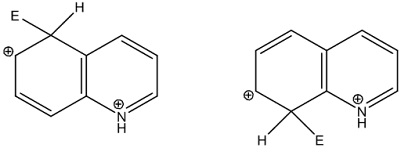
2) Electrophilic
substitution: electron rich nitrogen atom is main center for attack of
electrophiles
• Also heteroatom has considerable deactivating effect on
ring towards electrophilic attack
• It requires severe conditions though less than pyridine
• C-5 and C-8 positions are preferred for electrophilic
attack
• Halogenation:
depends on the nature of reagent employed
• Chlorination
(SO2Cl2) yields 3-chloroquinoline
• Bromination (Br2,
CCl4) yields 3-bromoquinoline
• In the presence of strong acids (Br2, Ag2SO4)
give a mixture of 5- and 8-bromoquinolines
• Reaction with
nucleophiles: attack by nucleophile occurs on pyridine ring of quinoline
and position-2 is preferred
• If position-2 is occupied the attack may takes place at
4-position
• Chichibabin reaction is example of normal attack at C-2
Chemistry
of Isoquinolines
• Obtained by the fusion of pyridine with a benzene ring
• First isolated by Hoogewerff and Dorp from the quinoline
fraction of coal tar in 1885
• Isoquinoline doesn’t occur free in nature but abundantly
in several alkaloids
• It is called as 2-azanaphthalene or benzo [c] pyridine
• Numbering same as quinoline, but the nitrogen atom is
assigned postion-2
• Because of similar in structure, both show close
relationship in physical and chemical properties
Physical properties of Isoquinolines:
• Colorless solid with melting point 243 0C
• Smell resembling that of benzaldehyde
• Steam volatile and sparingly soluble in water but soluble
in most organic solvents
• Isoquinoline turns yellow on keeping
• Isoquinoline is highly aromatic
• Weak base with pKa 5.14
• Electrophilic attack is preferred at C-5 and C-8 positions
Synthetic methods of Isoquinoline:
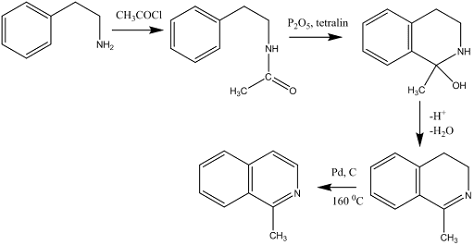
The Bischler-Napieralski synthesis:
• Involves a cyclodehydration of an acyl derivative of β-phenylethylamine to give
3,4-dihydroisoquinoline in the presence of polyphosphoric acid, zinc chloride
or phosphorus pentoxide
• Then its dehydrogenated to isoquinoline
Chemical properties of Isoquinoline:
Electrophilic substitution: takes place under rather
drastic conditions than pyridine
• 5- and 8- positions are mostly preferred
Halogenation:
• Chlorination (Cl2, AlCl3) gives
5-chloroisoquinoline in a low yield
• Bromination (Br2, AlCl3) takes place
at 5-position
Reaction with nucleophiles: attack of nucleophile
takes place at position-1
• If its occupied then attack occurs at 3-position
• Chichibabin reaction is an example of normal attack at C-1
Summary
• Quinoline structure obtained by ortho-condensation of
benzene ring with pyridine
• Highly aromatic
• Pyridine ring in quinolone is π-electron deficient,
therefore nucleophilic attack takes place at 2- and 4- position
• Chichibabin reaction is an example of normal attack at C-2
• Electrophilic attack preferably takes place at 5- and 8-
positions
• Isoquinoline is obtained by the fusion of pyridine with a
benzene ring
• Electrophilic attack is preferred at C-5 and C-8 positions
• Chichibabin reaction is an example of normal attack at C-1