Chemistry of Acridine
Session Objectives
By the end of this
session, students will be able to:
• Discuss chemistry, reactivity, method of synthesis of
acridine.
ACRIDINE
• Carl Grabe and Heinrich Caro first isolated acridine in
1870 from coal tar
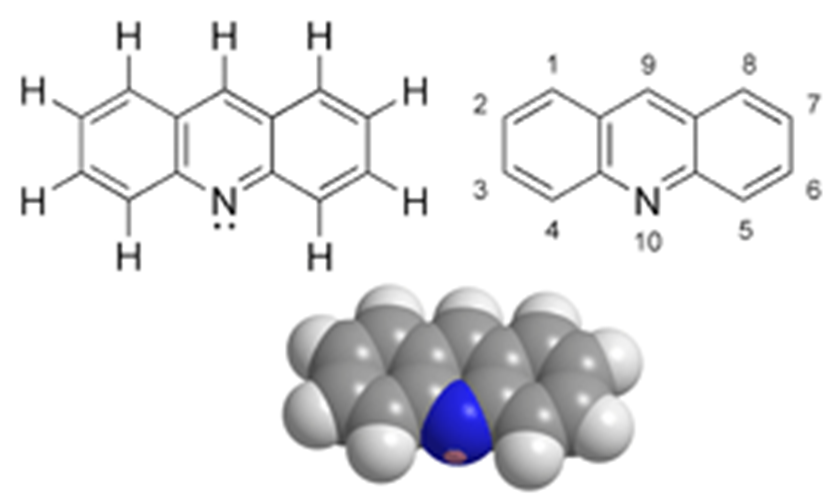
• It is an aza derivative of anthracene.
• Nitrogen atom is assigned position 10
• Acridine and its derivatives have applications as
chemotherapeutic drugs and dye stuffs.
PROPERTIES
• Pale
yellow crystalline solid.
• Melting
Point: 114 C
• Acridines
and its derivatives gives fluorescent solution.
Basicity
• Acridine
and its homologues are weakly basic. Acridine has a pKa of 5.1 similar to that
of pyridine.
SYNTHESIS OF ACRIDINE
• The Bernthsen
acridine synthesis is the chemical reaction of a diarylamine heated with a
carboxylic acid (or acid anhydride) and zinc chloride to form a 9-substituted
acridine
• Using
zinc chloride, one must heat the reaction to 200-270 °C for 24hrs. The use
of polyphosphoric acid will give acridine products at a lower temperature, but
also with decreased yields.
CHEMICAL REACTIONS OF ACRIDINE
• Reaction
with acids: Acridine
is a weak base but forms soluble salts with mineral acids.
Electrophilic
Substitution:
• Bromination
gives 2 and 2,7-dibromo products.
• Acridine
N-oxide undergoes nitration (HNO3/H2SO4 at OC) to produce 9-nitro acridine
N-oxide.
Reaction
with Nucleophilic reagents:
• It
undergoes nucleophilic attack at position 9.
• The
decreased electron density at this position when compared to 1,2,3,4
• Reaction
of acridine with sodium amide in liquid ammonia gives 9-aminoacridine.
Reaction
with oxidising agent:
• Acridine
is a very stable ring system towards the action of oxidising agents.
• It is not
easily oxidised but is converted into acridine N-oxide with peracids.
• This
oxide is a yellow solid. MP: 169 and in ethanol solution gives a green
fluorescein.
• It is
brominated and nitrated at position 9.
Reaction
with Reducing agents: 9-substituted acridines can be reduced by
• Sodium in
ether to corresponding acridanes.
• The
benzene ring in acridine is selectively reduced by pd or Rh/C in hydrochloric
acid, platinum oxide in trifluoro acetic acid to produce dioctahydro acridine.
Applications of Acridine
• Several
dyes and drugs feature the acridine skeleton.
• Many acridines,
such as proflavine, also have antiseptic properties.
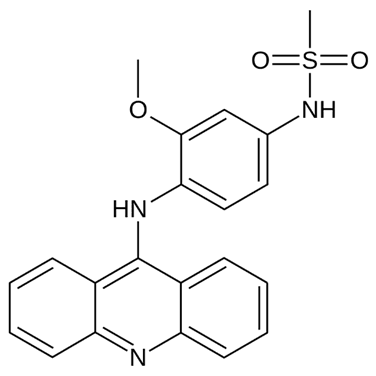
• Acridine
and related derivatives (such as amsacrine) bind to DNA and RNA due to their
abilities to intercalate. It has been used in acute lymphoblastic leukemia.
• Acridine
orange (3,6-dimethylaminoacridine) is a nucleic acid-selective metachromatic
stain useful for cell cycle determination.