Chemistry of Pyrimidine
Session Objectives
By the end of this
session, students will be able to:
• Discuss the chemistry, reactivity, properties and method
of synthesis of Pyrimidine
Chemistry
of Pyrimidine
• Most important member occurs widely in living organisms
• Purines, uric acid, alkoxan, barbituric acid
• First isolated by Gabriel and Colman 1899
• Pyrimidine is symmetrical about line passing C-2 and C-5
• Positions C-4 and C-6 are equivalent and so are N-1 and
N-3
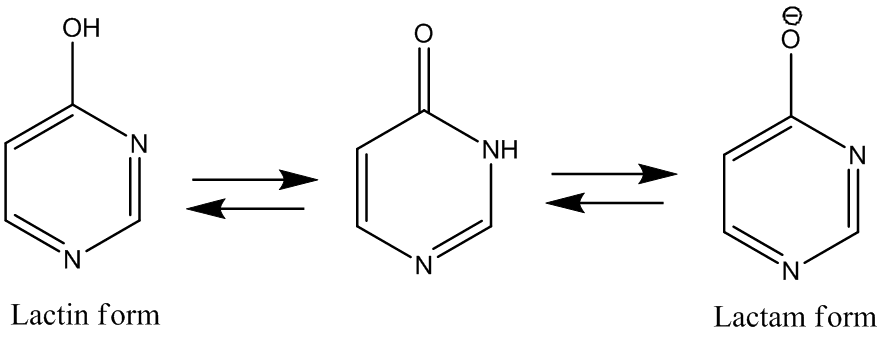
• When a hydroxy or amino group is present at 2-, 4-, or 6-
position then they are tautomeric with oxo and imino
Physical properties of Pyrimidine:
• Colorless compounds with melting point 225 0C,
boiling point 124 0C
• Weakly basic with pKa 1.3 due to electron-withdrawing
effect of 2nd nitrogen atom present in the ring
• Presence of alkyl groups increase the basicity,
4-methylpyrimidine pKa-2.0, 4,6-dimethylpyrimidine pKa 2.8, 2- and
4-aminopyrimidines are more basic with pKa 3.54 and 5.71
Synthetic methods of Pyrimidine:
Most important method is in which ring is formed from two
fragments which contribute C-C-C and N-C-N atoms
1) From
malonic esters:
• Involves condensation between a malonic ester and urea in
the presence of base to yield barbituric acid
2) From malic
acid:
• α-formyl acetic acid formed insitu by
decarboxylation of malic acid with conc. Sulfuric acid and then
subsequent reaction of β-keto acid(α-formyl acetic
acid) with urea to form uracil.
• Then Uracil can be converted to pyrimidine by chlorination
and reduction
Chemical reactions of Pyrimidine:
1) Reaction
with acids:
• Though a weak base can be protonated in the presence of
acids
• Diprotonation takes place in strong acids- possible
because nitrogen atoms are not present in adjacent positions
2) Electrophilic
substitution:
• Also resistant to electrophilic substitution
• Attack at 2-, 4-, 6- positions is particularly retarded
because of electron deficiency
• 5- position also difficult because of inductive effect of
two nitrogens
• Electrophilic substitution at 5- position becomes easy
when one or more electron releasing groups are present on the ring
• Bromination of pyrimidine yields 5-bromopyrimidine
3) Reaction
with nucleophilic reagents:
• Attack of nucleophile takes place easily
• Positions susceptible to attack are 2-, 4- and 6-.
• Stable in cold alkali but in boiling hydrazine it
rearranges to pyrazole, via a ring opened intermediate
• Grignard and organo-lithium reagents readily add to 3,4-
bond of pyrimidines at room temperature- phenyl magnesium bromide
• Intermediate on hydrolysis and subsequent oxidation yields
4-phenylpyrimidine
Summary
• Most important member occurs widely in living organisms
• Pyrimidine is symmetrical about line passing C-2 and C-5
• Positions C-4 and C-6 are equivalent and so are N-1 and
N-3
• Electrophilic attack at 2-, 4-, 6- positions is
particularly retarded because of electron deficiency