Clemmensen Reduction & Birch Reduction
Session Objectives
By the end of this
session, students will be able to:
• Clemmensen Reduction
• Mechanism of
clemmensen reduction
• Birch Reduction
• Mechanism of
birch reduction
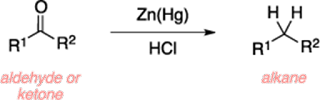
Clemmensen reduction
• Clemmensen reduction is an organic reaction used to reduce
an aldehyde or ketone to an alkane using amalgamated zinc and hydrochloric
acid.
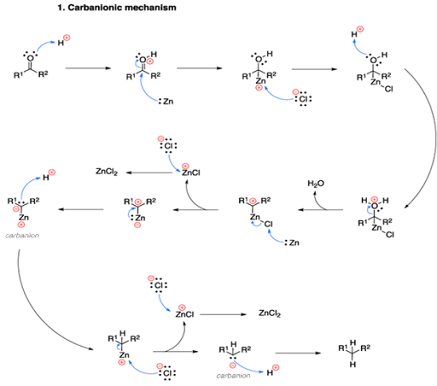
• The “Carbanionic mechanism”, where the zinc
attacks the protonated carbonyl directly, and the “Carbenoid
mechanism” which is a radical process and the reduction happens on the
surface of the zinc metal.
Mechanism of Clemmensen reduction
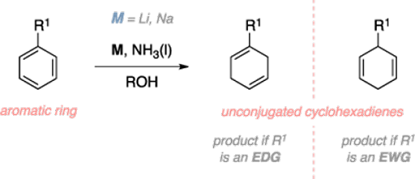
Birch reduction
• The Birch reduction is an organic reaction where aromatic
rings undergo a 1,4-reduction to provide unconjugated cyclohexadienes.
• The reduction is conducted by sodium or lithium metal in
liquid ammonia and in the presence of an alcohol.
• The mechanism begins with a single electron transfer (SET)
from the metal to the aromatic ring, forming a radical anion.
• The anion then picks up a proton from the alcohol which
results in a neutral radical intermediate.
• Another SET, and abstraction of a proton from the alcohol
results in the final cyclohexadiene product and two equivalents of metal
alkoxide salt as a byproduct.
Mechanism of Birch reduction
Summary
• Clemmensen reduction is an organic reaction used to reduce
an aldehyde or ketone to an alkane using amalgamated zinc and hydrochloric
acid.
• The “Carbanionic mechanism”, where the zinc
attacks the protonated carbonyl directly, and the “Carbenoid
mechanism”, which is a radical process and the reduction happens on the
surface of the zinc metal.
• The Birch reduction is an organic reaction where aromatic
rings undergo a 1,4-reduction to provide unconjugated cyclohexadienes.
• The reduction is conducted by sodium or lithium metal in
liquid ammonia and in the presence of an alcohol.
• The mechanism begins with a single electron transfer (SET)
from the metal to the aromatic ring, forming a radical anion.