Enantiomers, Diastereomers & Meso Compounds
Session Objectives
By the end of this
session, students will be able to:
• Explain configuration of enantiomers
• Classify Enantiomers and Diastereomers
• Assign D and L system to isomers
• Define meso compounds
Optical activity
• A solution of optically active molecule (enantiomer) is
placed in a sample tube, plane-polarized light is passed through the tube and a
rotation of the polarization plane takes place
• The light then goes through a second polarizer called an
analyser
• By rotating the analyser until the light passes through
it, the new plane of polarization can be found, and the extent of rotation that
has taken place can be measured
• A mixture of enantiomers with the same amount of each is
called a racemic mixture
• Racemic mixtures are optically inactive (i.e. they cancel
each other out) and are denoted by (±)
Note: the amount
of rotation depends on sample concentration and sample path length
• To obtain a meaningful optical rotation data, we have to
choose standard conditions
• The specific rotation of a compound, designated as [α]D, is defined as the
observed rotation, when the sample path length l is 1 dm, the sample
concentration C is 1g/mL and light of 599.6 nm wavelength (the D line of a
sodium lamp, which is the yellow light emitted from common sodium lamps) is
mostly used
• As the specific rotation also depends on temperature, the
temperature at which the rotation is measured and denoted more precisely as
Enantiomers can be described as (+) or (-)
• We can use the fact that two enantiomers rotate
plane-polarized light in opposite directions to assign each a label that
doesn’t depend on knowing its configuration
• We call the enantiomer that rotates plane-polarized light
to the right (gives a positive rotation) the (+)-enantiomer (or the dextrorotatory enantiomer)
• And the enantiomer that rotates plane-polarized light to
the left (gives a negative rotation) the (–)-enantiomer (or the laevorotatory enantiomer)
Enantiomers and Diastereomers
• In general molecule with n chiral centers, the maximum
number of stereoisomers possible is 2n
• That means for one chiral center its 2, for 2 its 4, for 3
its 8 and so forth
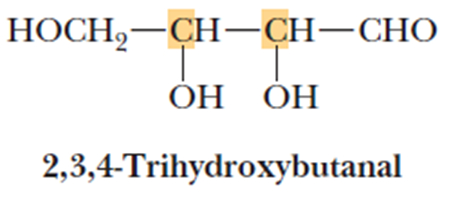
• Let us consider 2,3,4-trihydroxybutanal
• In general molecule with n chiral centers, the maximum
number of stereoisomers possible is 2n
• Let us consider 2,3,4-trihydroxybutanal
• It contains two chiral centers and 4 stereoisomers are
possible
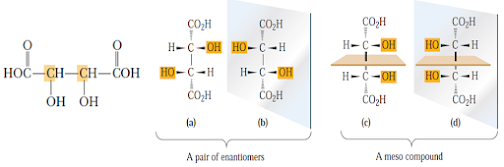
• Stereoisomers (a) and (b) are nonsuperposable mirror
images and are, therefore, a pair of enantiomers
• Stereoisomers (c) and (d) are also nonsuperposable mirror
images and are a second pair of enantiomers
• On naming, enantiomers (a) and (b) as (2R,3R)-erythrose
and (2S,3S)-erythrose; enantiomers (c) and (D) as (2R,3S)-threose and (2S,3R)-threose
• Both belongs to the class of carbohydrates and erythrose
is present in erythrocytes (red blood cells)
• What is the relationship between (a) and (c), (a) and (d),
(b) and (c), (b) and (d)?? Answer is diastereomers
• Diastereomers are the stereoisomers that are not mirror
images
• Molecules with at least two chiral centers can have
diastereomers
Meso compounds
• Molecules containing two or more chiral centers have
special symmetry properties that reduce the number of stereoisomers to fewer
than the maximum number predicted by the 2n rule
• For example, 2,3-dihydroxybutanedioic acid commonly called
as tartaric acid
• In tartaric acid, carbons 2 and 3 are chiral centers,
number of stereoisomers possible is 4
• (a) and (b) are nonsuperposable mirror images, enantiomers
• (c) and (d) are superposable mirror images, meso compounds
• Also (c) has plane of symmetry and is achiral
• A meso compound contains two or more chiral centers and is
achiral
• To be a meso compound, molecule must also have chiral
isomers
• Hence, tartaric acid has three stereosisomers, one pair of
enantiomers and a meso compound
• Enantiomers of tartaric acid will have same melting point,
boiling point, solubility in water and other common solvents, same value of pKa,
and they undergo the same acid-base reactions
• But differ in optical activity
• Diastereomers have different physical and chemical
properties, even in achiral environments
• Meso tartaric acid has different physical properties from
those of the enantiomers and can be separated from them by methods such as
crystallization
Physical properties of tartaric acid
Summary
• Enantiomer that rotates plane-polarized light to the right
(gives a positive rotation) the (+)-enantiomer (or the dextro-rotatory enantiomer)
• Enantiomer that rotates plane-polarized light to the left
(gives a negative rotation) the (–)-enantiomer (or the laevo-rotatory
enantiomer)
• The direction in which light is rotated is not dependent
on whether a stereogenic centre is R or S
• Optical activity does not tell us the actual configuration
of an enantiomer
• In general molecule with n chiral centers, the maximum
number of stereoisomers possible is 2n
• Diastereomers are the stereoisomers that are not mirror
images
• Molecules with at least two chiral centers can have
diastereomers
• A meso compound contains two or more chiral centers and is
achiral