Lithium Aluminium hydride reduction
Session Objectives
By the end of this
session, students will be able to:
• Structure of lithium Aluminium hydride
• Properties of lithium Aluminium hydride
• Reactions of lithium Aluminium hydride
Lithium aluminium hydride
• Lithium aluminium hydride, LiAlH4, also abbreviated as
LAH, is a reducing agent commonly employed in modern organic synthesis.
• It is a nucleophilic reducing agent, best used to reduce
polar multiple bonds like C=O.
• LiAlH4 can reduce aldehydes to primary alcohols, ketones
to secondary alcohols, carboxylic acids and esters to primary alcohols, amides
and nitriles to amines, epoxides to alcohols and lactones to diols.
• Lithium aluminium hydride cannot reduce an isolated
non-polar multiple bond like C=C. However, the double or triple bonds in
conjugation with the polar multiple bonds can be reduced.
• LiAlH4 is a powerful reducing agent compared to sodium
borohydride, NaBH4, since the Al-H bond is weaker and thus less stable than B-H
bond.
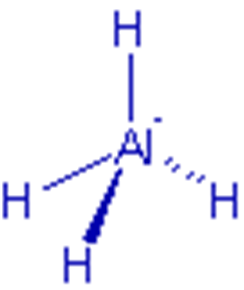
Structure of Lithium aluminium
hydride – LiAlH4
• There is a tetrahedral arrangement of hydrogens around
aluminium in aluminium hydride, AlH4– ion.
• It is formed by coordination of hydride, H–
ions to aluminium, Al3+ ion.
• The hybridization in central aluminium is sp3.
Preparation
• LiAlH4
is prepared by the reaction between lithium hydride and aluminium chloride.
Properties
• The reaction must be carried out in anhydrous non protic
solvents like diethyl ether, THF etc.
• It is highly soluble in diethyl ether.
• However it may spontaneously decompose in it due to
presence of catalytic impurities.
• Therefore the preferred solvent for LAH is THF
despite the low solubility.
• The reactions are usually performed with excess of LiAlH4.
• White
solid but the commercial samples are usually gray due to presence of
impurities.
• *It
reacts violently with water by producing hydrogen gas.
• Hence it
should not be exposed to moisture and the reactions are performed in inert and
dry atmosphere.
Workup:
• During the workup, the reaction mixture is initially
chilled in an ice bath and then the Lithium aluminium hydride is quenched by
careful and very slow addition of ethyl acetate followed by the addition of
methanol and then cold water.
• Sometimes, the reagent is decomposed by adding undried
solvent slowly and then dilute sulphuric acid to the reaction mixture.
Mechanism of Reduction By
Lithium Aluminium Hydride
• The
reduction of a carbonyl group by LiAlH4 is initiated by the attack
of nucleophilic hydride ion on the carbonyl carbon to give a tetrahedral
intermediate.
• * LiAlH4
is a nucleophilic reducing agent since the hydride transfer to the carbonyl
carbon occurs prior to the coordination to the carbonyl oxygen.
• It reacts
faster with electron deficient carbonyl groups.
• The
reactivity of carbonyl compounds with this reagent follows the order:
Aldehydes
> Ketones > ester > amide > carboxylic acid
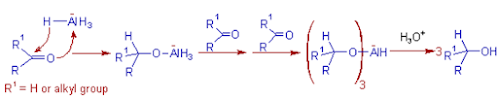
Reduction
of Aldehydes or Ketones to 10 or 20 alcohols: Initially, a hydride ion is
transferred onto the carbonyl carbon and the oxygen atom coordinates to the
remaining aluminium hydride species to furnish an alkoxytrihydroaluminate ion,
which can reduce the next carbonyl molecule. Thus three of the hydride ions are
used up in reduction.
Reduction
of Esters to 10 alcohols: The ester is first converted to aldehyde which
is further reduced to primary alcohol.
Reduction of Amides to amines:
• Amides
are converted to amines.
• The
mechanism is slightly different from that depicted for esters.
• In
iminium ion is formed during the reaction since nitrogen atom is relatively a
good donor than oxygen atom.
Reduction of nitriles to
primary amines:
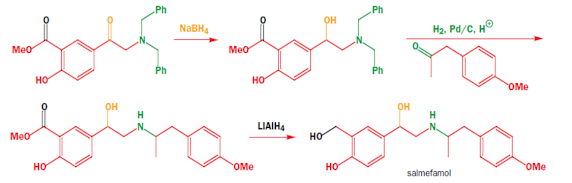
Synthesis of Salmefamol
Summary
• It is a nucleophilic reducing agent, best used to reduce
polar multiple bonds like C=O.
• LiAlH4 can reduce aldehydes to primary alcohols, ketones
to secondary alcohols, carboxylic acids and esters to primary alcohols, amides
and nitriles to amines, epoxides to alcohols and lactones to diols.
• Lithium aluminium hydride cannot reduce an isolated
non-polar multiple bond like C=C.
• However, the double or triple bonds in conjugation with
the polar multiple bonds can be reduced.