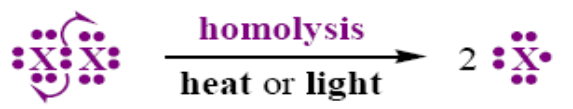Reaction Intermediates
Contents
• Heterocyclic fission
• Homolytic fission
• Bond dissociation energy
• Nucleophiles
• Electrophiles
• Nucleophiles and electrophiles
• Carbocation
• Stability of carbocation
Learning Objectives
At the
end of this lecture, student will be able to
• Define the terms
• Heterocyclic fission
• Homolytic fission
• Bond dissociation energy
• Nucleophiles
• Electrophiles
• List examples for nucleophiles and
electrophiles
• Define the term carbanion
,free radicals , carbenes and nitrenes
• Discuss the generation and fate of
carbanions and free radicals
• Compare the stabilities of different carbanions
• Compare the stabilities of different
free radicals
Different Types of Reaction
AB+CD à AC+BD
• Elimination reaction
Aà B + C
• Addition reaction A + B à C
• Rearrangement reactions A à B
Definitions
Bond dissociation energy
• Measure of the strength in
a chemical bond
• It is defined as the standard
enthalpy change when a bond is cleaved
Heterolytic fission
• If a bond were to split unevenly
(one atom getting both electrons, and the other none), ions would be formed
• The atom that got both electrons
would become negatively charged
• While the other one would become
positive
Homolytic fission
• If the bond breaks in such a way
that each fragment gets one electron
• Free radicals are formed
Nucleophile
• Nucleus loving
• Electron rich
• Attacking reagents
Types of nucleophile
• Negative nucleophile
• Neutral nucleophile
• Negative
nucleophile, Examples: Cl‾, OH‾, CN‾, OR‾, H‾, X‾, SH‾
• Neutral nucleophile, Examples:
NH3, H2O, ROH, RSH, ROR
Electrophile
• Electron loving species
Types
• Neutral, Examples: BF3,
AlCl3
• Positive electrophiles, Examples:
Carbocation Br+
Reaction Intermediates
• Reactions in organic chemistry
proceed in more than one step
• Via one more short lived reactive
intermediate
• Starting material à Intermediate à Products
• Specific sequence in which bonds are
made and broken
• Reactants are converted into
products
• Carbocation
• Carbanion
• Free radicals
• Carbenes
• nitrenes
Carbocation
•
Electron
deficient
• Positively charged reaction intermediate
• Formed by heterolysis of covalent bond
• Generation
Classification
Primary carbocation 1 alkyl substituent bonded to positively charged
carbon
Secondary carbocation 2 alkyl substituents
Tertiary carbocation 3
alkyl substituents
Examples
Stability of Carbocations
• Inductive effect
• Hyperconjugative effect
Inductive effect
• G
→ electron releasing group like alkyl group → stabilizes carbocation
• G
→ electron withdrawing group like nitro group → destabilizes carbocation
•
Hyperconjugation
•
Order of stability of carbocations
Rearrangement
of Carbocations
•
Driving force – stability
•
1° carbonium ion
rearrange to a 2° or 3° carbonium ion
CH3-CH2-CH2-CH2+
———→ CH3-CH2-CH+-CH3
CH3 CH3
ǀ
ǀ
CH3-CH2-CH-CH2+
———→ CH3-CH2-C-CH3
•
2° carbonium ion rearrange to 3° carbonium ion
CH3 CH3
ǀ ǀ
CH3-C-CH-CH3 ———→ CH3-C-C-H
ǀ + + ǀ
CH3 CH3
•
Rearrangement takes place by 1,2-shift
•
Migration of hydrogen – hydride shift
•
Migration of alkyl group – alkyl shift
1,2 – hydride shift
1,2 – methyl shift
Carbanion
• Carbon atom bearing negative charge
• Typically nucleophile and basic in
nature
Stability
of Carbanions
• Basicity
and nucleophilicity of carbanions are determined by the substituents on carbon
• Inductive
effect Electronegative atoms adjacent to the charge will stabilize the charge;
• Extent
of conjugation of the anion. Resonance effects can
stabilize the anion
• Conjugation with unsaturated bond
• Order of stability
• Because of fact that greater
s-character, closer are the electrons to the nucleus and hence of lower
energy
Fate of Carbanions
• Addition reaction
Reaction intermediates
• Of
the five, only carbanions have complete octet around carbon
Free Radical
• Species which is having odd or unpaired electron
• Highly reactive species
Stability
• Hyperconjugative effect
• Resonance effect
• Relative stabilities of free
radicals
• Benzyl >allyl >tert > sec
> primary > methyl
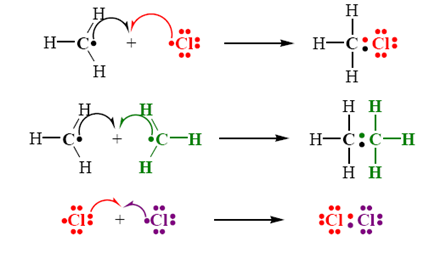
Generation
of Free Radicals
• Free radicals are formed from
molecules by breaking a bond so that each fragment keeps one electron
• Energy necessary to break the bond
is supplied in two ways
• Thermal cleavage or photochemical cleavage of covalent bonds
• Reactions of free radicals either
give stable products or lead to other radicals
Fate of
Free Radicals
• Radical –radical interaction
• Radical –molecule interaction
(propagation reactions)
• Addition to multiple bonds
Carbenes
• Carbenes are neutral species
• Carbon atom with two bonds and two electrons
• R2C:
Generation of carbenes
• In
a elimination, a carbon loses a group without its electron pair, usually a
proton, and then a group with its pair, usually a halide ion
• Formation
of dichlorocarbene by treatment of chloroform with base
• Photolysis
of ketene
Fate of carbenes
• Addition
to carbon-carbon double bonds
• Addition
to aromatic systems- usually with ring enlargement
• Carbene
reacts with methane to give ethane and propane
Nitrenes
• Nitrogen analogue of carbene
• Electron deficient species
• Nitrogen has six electrons
• RN:
• Too reactive for isolation under
ordinary conditions
Generation of nitrenes
• Breakdown
of certain double bond compounds
• Photolytic
or thermal decomposition of azides
Fate of nitrenes
• Addition
to carbon-carbon double bonds
• Rearrangements
of alkyl nitrene
Summary
• Products of homolytic fission are
free radicals
• Products of heterocyclic fission are
ions
• Intermediates are highly unstable
• They are formed in a rection
• Carbocations, carbanions, free
radicals, carbene and nitrenes are
reaction intermediates
• Carbanions are negatively charged
species
• Carbanions are formed by heterolytic
fission of bonds
• Carbanions undergo addition reactions
• Tertiary carbanion is the least
stable anion
• Free radicals are neutral species
and possess an odd electron
• Free radicals are formed by
homolytic fission of bonds
• Free radicals are formed by Thermal
cleavage or photochemical cleavage
of covalent bonds
• Free radicals take part in Radical
–molecule interaction (propagation reactions) and addition to multiple bonds
• Benzyl free radical is the most
stable free radical
• Carbon with two electrons and two
bonds are called as carbenes
• Nitrogen analogue of carbene is
called as nitrene