Electron Displacement Effects
Contents
• Inductive effect
• Resonance effect
• Electromeric effect
• Examples
Learning
Objectives
At the end of this
lecture, student will be able to
• Explain different electron displacement effects
• Electron Displacement effects
• Inductive effect
• Resonance effect
• Electromeric effect
Factors
effecting reactivity
• Steric
effects- concerned with the size and shape of groups within molecules
• Electronic
effects- result from the electronegativity differences between atoms affect the
way electrons are distributed in molecules
• Can be
divided into inductive and mesomeric effects
• Inductive
effects- consequence of way that electronegativity differences leads to
polarization of σ bonds
• Mesomeric
effects- affects the distribution of electrons in π bonds
• Inductive
effect- polarization of σ bond
• Cause-
electronegativity difference between the atoms
• Creates
some bond polarity between atoms
• Most
electronegative atoms pulls electrons in the bond towards itself which results
in polarization of bond
• It is a
permanent effect
• It
influences physico-chemical properties
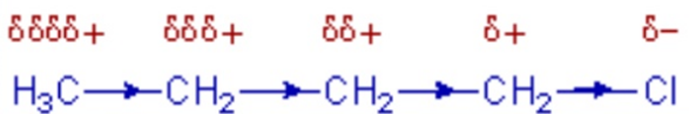
Inductive
effect
• Inductive
effect weakens away along the chain and is not significant beyond 3rd
carbon atom
• Types of
Inductive effect
• Negative
inductive effect (-I)
• Positive
inductive effect (+I)
• Electron
withdrawing nature of groups or atoms is called negative inductive effect
• Electron-withdrawing
groups include halogen, nitro, cyano, carboxy, ester and aryloxy
• Positive
inductive effect- refers to electron releasing nature of groups or atoms
• Alkyl
group are usually considered as electron donating groups
• Why alkyl
groups are showing positive inductive effect?
• Though
the C-H bond is practically non-polar covalent bond, there is partial positive
charge on hydrogen atom
• And
partial negative charge on carbon atom
• Each
hydrogen acts as electron donating group and turns alkyl moiety into electron
donating group
Applications of inductive
effect
• Stability
of carbocations
• Increases
with the increase in number of alkyl groups
• Due to
the +I effect
• Alkyl
groups releases electrons to carbon bearing positive charge and stabilizes ion
• Stability
of carbanions
• Decreases
with the increase in number of alkyl groups
• Electron
donating groups destabilize the carbanions by increasing the electron density
• Acidic
strength of carboxylic acids and phenols
• Electron
withdrawing groups decreases the negative charge on carboxylate ion and
stabilizes it
• Acidic
strength increases when –I groups are present
• p-nitro
phenol is more stronger than phenol
• Because
nitro group is –I group and withdraws
electron density
• p-cresol
is weaker acid, because methyl group is +I effect
• Basic
strength of amines
• Electron
donating groups increases the basic strength of amines
• Electron
withdrawing groups decreases the basic strength
• Alkyl
amines are stronger than ammonia
• Aryl
amines are weaker than ammonia
• For
example, CH3NH2,NH3,C6H5NH2
• Ans: CH3NH2>NH3>C6H5NH2
Problem-01
• Arrange it in the order of acidic strength
A) CH3COOH, CH2FCOOH, CHF2COOH, CF3COOH
Ans: CH3COOH<CH2FCOOH<CHF2COOH<CF3COOH
B) HCOOH, CH3COOH
Ans: Formic acid is stronger than acetic acid
• -CH3 destabilizes the carboxylate ion
Resonance
Effect /Mesomeric Effect
• Also
called as resonance effect
• Arises
due to substituents or functional groups in a molecule
• Represented
by letter M or R
• Polarity
produced in the molecule by the interaction of two π bonds or between a π bond and lone pair of electrons
present on adjacent atom
• Negative
mesomeric effect
• Shown by
substituents or groups that withdraw electrons
• Denoted
by –M or –R effect
• Electron
density on rest of the molecule will be decreased by this
• For
example, -NO2, carbonyl group C=O, cyano, -COOH, -SO3H,
etc
• Positive
mesomeric effect
• Shown by
substituents or groups that donates electrons
• Denoted
by +M or +R effect
• For
example, -OH, -OR, -SH, -SR, -NH2, -NR2
Applications of mesomeric
effect
• Nitro
group in nitrobenzene shows –M effect
• Electron
density on benzene ring is decreased particularly on ortho and para positions
• Nitro
group deactivates the benzene ring towards electrophilic substitution reaction
• In
phenol, -OH group shows +M effect due to delocalization of lone pair of
electrons on oxygen atom towards the ring
• Electron
density on benzene ring is particularly increased on ortho and para positions
• Hence
phenol is more favoured towards electrophilic substitution
• Also more
favoured at ortho and para positions
• -NH2
in aniline also exhibits +M effect
• Releases
electrons towards benzene ring through delocalization
• By this
electron density on benzene ring increases particularly at ortho and para
positions
• Aniline
activates towards electrophilic substitution
• Causes
less basic than ammonia and alkyl amines
Electromeric
effect
• Electromeric effect is a temporary effect
• The polarization of pie bond by a nucleophile to form
temporary addition compound
• Can be regained if the attacking species is expelled out
from it by adding some strong electrophile
Hyperconjugative Effect
• Stabilising
interaction that results from the interaction of the electrons in a σ-bond
(usually C-H or C-C) with an adjacent empty or
partially filled p-orbital or a π-orbital
• To give
an extended molecular orbital that increases the stability of the system
• For example,
hyperconjugation in carbocation centre
• C-H bond
• Conjugative effect
• Through sigma and pi bonds
• In
resonating structures of propene, there is no bond between carbon and hydrogen
atom
• Hyperconjugation
also called as no bond resonance
• Number of
methyl groups bonded in double bonded carbon atom increases, possibility of
hyperconjugation increases
• Results
more stability- more substituted alkenes are more stable than less substituted
alkenes
Summary
• Inductive effect occurs through sigma bond
• Inductive effect is a permanent effect
• Mesomeric effect is a temporary effect
• Hyperconjugative
effect is through sigma and pi bonds