Elimination of Drugs
Elimination of Drugs
Drug Elimination
• Termination of drug effect is by the process of drug elimination which involves mainly two processes:
1. Metabolism: predominantly in the liver and kidney.
2. Excretion of unchanged drug or its metabolite predominantly by kidney.
§ Excretion also takes place in the gut, skin, lungs, sweat glands, breast and salivary glands.
3. In addition there is a third process of termination of drug action i.e. process of redistribution of drug.
• The onset of pharmacological response depends on:
1. Drug absorption
2. Drug distribution
• The duration and intensity of action depends upon:
1. Tissue redistribution of drug and
2. The rate of elimination
Introduction
“Excretion is defined as the process whereby the drugs and/or their metabolites are irreversibly transferred from internal to external environment.”
Types of Excretion
1. Renal excretion
2. Non-renal excretion
– Lungs.
– Biliary system.
– Intestine.
– Salivary glands.
– Sweat glands.
Agents that are excreted in urine are-
– Water soluble
– Non-volatile
– Small in molecular size (<500 daltons)
– The ones that are metabolized slowly.
The principle processes that determine the urinary excretion of a drug are-
– Glomerular filtration.
– Active tubular secretion.
– Active or passive tubular Reabsorption.
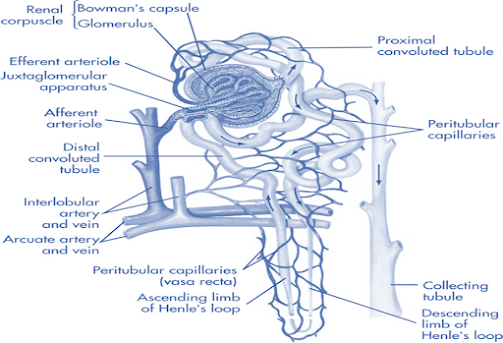
Renal Excretion
Rate of Excretion= (Rate of Filtration + Rate of Secretion) – (Rate of Reabsorption)
Glomerular filtration:
• Non-selective, unidirectional.
• Most compounds, ionized or unionized are filtered except those that are bound to plasma proteins or blood cells, thus behaving as macromolecules.
• The driving force for filtration through the glomerulus is the hydrostatic pressure of the blood flowing in the capillaries.
• The glomerulus acts as a negatively charged selective barrier promoting retention of anionic compounds.
• Substances used for determination of GFR:
– Creatinine.
– Inulin.
– Mannitol.
– Sodium thiosulphate.
Active tubular secretion:
• Carrier mediated, capacity limited, saturable.
• Occurs in proximal tubule of nephron.
• Requires energy for transportation of compounds against concentration gradient.
• Unaffected by pH & protein binding.
• Dependent on renal blood flow.
System for secretion of organic acids/anions:
e.g. penicillin, salicylates, glucuronides, sulphates & endogenous substances like Uric acid.
System for secretion of organic bases or cations;
e.g. Morphine, mecamylamine, hexamethonium, endogenous amines like catecholamines, choline, histamine.
• Two structurally similar drugs having similar ionic charge and employing the same carrier- mediated process for excretion enter into competition.
• A drug with greater rate of clearance will retard the excretion of other drug with which it competes.
• The half-life of both the drugs is increased since the total sites for active secretion are limited.
Competition
â
Increased half-life
â
Precipitation of
toxicity
• Therapeutic advantages of competition:
– Probenicid inhibits active tubular secretion of organic acids e.g. Penicillin, PAS, PAH,17-ketosteroids: increases their plasma conc. 2 fold.
– Probenecid acts as a uricosuric agent in treatment of gout.
à It suppresses the carrier mediated reabsorption of endogenous metabolite uric acid.
• Therapeutic disadvantages of competition:
Inhibition of
nitrofurantoin secretion by probenecid
â
Decreased efficacy
Tubular Reabsorption:
• Takes place all along renal tubule.
• Results in increases in half-life of a drug.
Active Tubular Reabsorption:
• Seen with high threshold endogenous substances or nutrients that the body needs to conserve.
e.g. glucose, electrolytes, vitamins, amino acids, uric acid, oxopurinol
Passive tubular Reabsorption:
• Concentration gradient is the prime driving force.
• Primary determinant: Lipophilicity.
• Lipophilic substances are extensively reabsorbed as compared to polar substances.
• Majority of drugs are weak electrolytes (weak acids or weak bases), so their re-absorption depends upon:
– pH of urine: (4.5-7.5)
– pKa of a drug: pKa values govern the degree of ionisation at a particular pH.
-Urine flow rate: Those drugs whose reabsorption is pH-sensitive, e.g. weak acids and weak bases, show dependence on urine flow rate.
1
Reabsorption α ——————————-
Urine Flow Rate
Factors Affecting Renal Excretion/Clearance
1. Physicochemical properties of the drug
2. Plasma concentration of the drug
3. Distribution and binding charectiristics of the drug
4. Urine pH
5. Blood flows to the kidneys
6. Biological factors
7. Drug interactions
8. Disease states.
1. Physicochemical Properties of Drug
• Molecular size
| Mol. Wt. | Excretion pattern |
| < 300 Daltons | Urine, < 5% in bile |
| >500 Daltons | Bile, <5% in urine |
| 300-500 | Both urine and bile |
• pKa
• Lipid solubility
– Urinary excretion is inversely related to lipophilicity.
• Stereochemical nature:
– Chloroquine, disopyramide & terbutaline are stereoselectively secreted by kidneys.
2. Plasma Conc. Of Drug
• Glomerular filtration and Reabsorption are directly affected by plasma concentration.
3. Distribution and binding characteristics of the drug
• Clearance is inversely related to apparent volume of distribution of drugs.
• A drug with large Vd is poorly excreted in urine.
• Drugs restricted to blood compartment have higher excretion rates.
4. Urine pH
• The pH of urine varies between 4.5 – 7.5, thus creating large pH gradient between urine and plasma.
• The pH of urine is dependent upon diet, drug intake and patho-physiology of patient.
• The relative amount of ionised and unionised drug in the urine at a particular pH and the % of drug ionised at this pH can be computed from the Henderson-Hasselbach equations.
• pH = pKa + log (Ionised drug/Unionised drug)…Weak acids
• pH = pKa + log (Unionised drug/Ionised drug)…Weak bases
• The therapeutic activity of urinary antiseptic examine depends upon urine pH. (Gets converted to active form formaldehyde at acidic pH)
5. Renal blood flow
• Important for drugs excreted by Glomerular filtration only and those are actively secreted.
• In case of drugs which are actively secreted: increased perfusion àincreased contact of drug with secretary site à increased excretion.
i.e. perfusion rate limited.
6. Biological factors
• Renal excretion is 10% lower in females.
• Newborns: 30-40% less (attains maturity between 2.5-5 months of age)
• Old age: – GFR decreased,
– Excretion decreased,
– Increased t1/2 .
7. Drug interactions
Alteration of Protein-Drug binding:
• The renal clearance of drugs extensively bound to plasma proteins is increased after displacement with another drugs. E.g. Gentamicin induced nephrotoxicity by Furosemide.. (Furosemide displaces gentamicin from protein)
Alteration of urine pH:
• Acidification of urine: by ammonium chloride, methionine and ascorbic acid à enhances excretion of basic drugs.
E.g. morphine, Amphetamine.
• Alkanisation of urine: by citrates, tartarates, bicarbonates and carbonic anhydrase inhibitors àenhances excretion of acidic drugs e.g. barbiturates, salicylates.
• Prevention of crystalluria caused by precipitation of sulphonamides in the renal tubules.
Competition for Active Secretion:
• Probenicid: competitive inhibitor of organic anion transport system.
• Cimetidine: competitive inhibitor of organic cation transport system.
Forced diuresis:
• All diuretics increases elimination of drugs whose renal clearance gets affected by renal blood flow rate.
8. Disease states
Renal dysfunction:
• Greatly impairs elimination of drugs primarily excreted by kidneys.
Disease states
Uraemia:
Impaired Glomerular
Filtration
â
Accumulation of Drugs
â
Toxicity.
Non-renal Routes of Drug Administration
Introduction
• Drugs and their metabolites may also be excreted by routes other than renal route, called as extrarenal or nonrenal routes of drug excretion.
• Biliary excretion.
• Pulmonary excretion
• Salivary excretion
• Mammary excretion
• Skin/Dermal excretion
• Gastrointestinal excretion
• Genital excretion
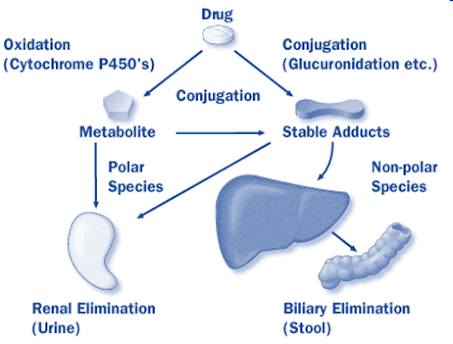
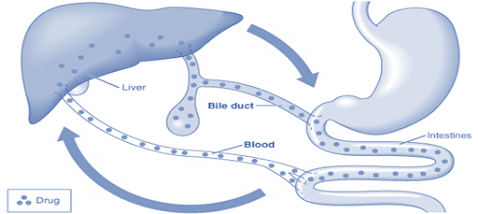
Biliary Excretion of Drugs -Enterohepatic Cycling
• Bile juice is secreted by hepatic cells of the liver.
• The flow is steady-0.5 to 1 ml /min.
• 90% of bile acid is reabsorbed from intestine and transported back to the liver for resecretion.
• Greater the polarity better the excretion.
• The metabolites are more excreted in bile than parent drugs due to increased polarity.
• Active process
• Capacity limited, saturable.
• E.g. Quinine, Cholchicine, Vinblastine, D-tubocurarine, Vecuronium, Corticosteroids, Erythromycin, Chlorpromazine
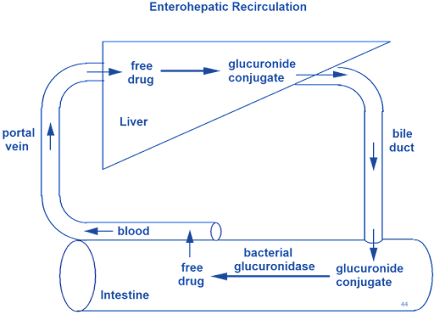
Enterohepatic Cycling / Enterohepatic Circulation of Drugs:
• “The phenomenon of drug cycling between the intestine and the liver.” e.g. cardiac glycosides, rifampicin, chlorpromazine, indomethacin.
• Increased t1/2 – e.g oral contraceptives, DDT.
• Prolongation of drug action:
e.g. Rifampicin – this drug is deacetylated by liver and both the free drug and deacetylated metabolite are excreted through bile into the gut from where rifampicin is reabsorbed while deacetylated metabolites is excreted through faeces. This drug acting as a small circulating reservoir à prolongation of drug action.
• Certain drug metabolites (particularly Glucuronides) are excreted through the bile and delivered to the intestine where these metabolites are deconjugated or hydrolysed releasing the parent active drug again.
• This free drug is then reabsorbed and the cycle is repeated. (Enterohepatic circulation)
E.g. Thyroxine, Morphine, Chloramphenicol, Phenophthalin, Ethynil Estradiol, Indomethacin (partially secreted as glucuronide)
Factors Influencing Secretion of Drugs in Bile:
1. Physicochemical properties of drug:
a. Molecular Weight:
| Mol. Wt. | Excretion pattern |
| < 300 Daltons | Urine, < 5% in bile |
| >500 Daltons | Bile, <5% in urine |
| 300-500 | Both urine and bile |
b. Polarity
Polarity α Excretion
• Thus metabolites are better excreted in bile rather than parent drug.
2. Nature of biotransformation process –
Metabolic reactions
â
Increased Polarity
â
Increased Mol.Wt
â
Favours Biliary Excretion of Metabolites.
e.g. phase II reactions
1. Glucuronidation: Morphine, Chloramphenicol, Indomethacin, Stilbesterol
2. Glutathione:
3. Other factors
• Sex and species differences
• Protein drug binding
• Disease states
• Drug interactions
Pulmonary Excretion
• Gaseous and volatile substances such as general anesthetics (Halothane) are absorbed through lungs by simple diffusion.
• Pulmonary blood flow, rate of respiration and solubility of substance effect Pulmonary Excreion. Intact gaseous drugs are excreted but not metabolites.
• Alcohol which has high solubility in blood and tissues are excreted slowly by lungs.
e.g. General anaesthetics – halothane, nitrous oxide etc.
Alcohol – excreted slowly through lungs.
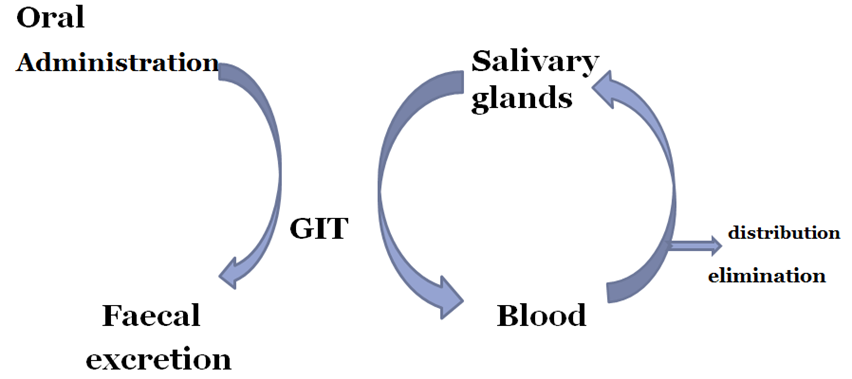
Salivary excretion
• The pH of saliva varies from 5.8 to 8.4.
• Unionized lipid soluble drugs are excreted passively.
• The bitter after taste in the mouth of a patient is indication of drug excreted.
• Some basic drugs inhibit saliva secretion and are responsible for mouth dryness.
• Passive diffusion process
• Dependent on pH partition hypothesis
• Basic drugs secreted more than acidic drugs
E.g. Lithium, Tetracycline, Metronidazole, Pyrizinamide, Penicillin, Ethosuximide, Phenytoin, Digoxin, Sulphonamide, Salicylates, Clonidine
• Estimation of blood levels of drugs like caffeine, theophylline, Phenytoin, carbamazepine Since the S/P ratio is fairly constant.
Salivary Cycling Of Drugs
• Drugs excreted in saliva can undergo cycling in a fashion similar to enterohepatic cycling e.g. sulphonamides, antibiotics, clonidine etc.
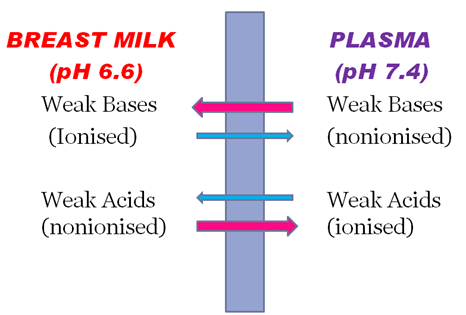
Mammary excretion
• Weakly basic drugs concentrate more in milk.
E.g. Chloramphenicol, tetracyclines, ergotamine, morphine, metronidazole, carbimazole, bromocriptine, diazepam estrogens/progesterone, Oral contraceptives, antihistaminics, senna alkaloids (purgatives)
| Drug | Effect |
| Chloramphenicol | Bone marrow depression |
| Diazepam | Accumulation and sedation |
| Heroin | Prolonged neonatal dependence |
| Methadone | Possible withdrawal symptoms if breast feeding is stopped suddenly |
| Propylthiouracil | Suppression of thyroid function |
| Drug | Effect |
| Tetracycline | Permanent staining on teeth |
| Sulphonamides | Kernicterus |
| Penicillin | Allergy |
| Ampicillin | Diarrhea |
| Dapsone | Hemolytic anemia |
| Phenindione | Bleeding |
| Phenobarbitone | Drowsiness |
| Phenytoin | Methhemoglobinemia |
| Theophylline | Restlessness |
Skin excretion (Sweat)
• Depends on pH partition hypothesis.
– Keratin precursor cells: Griseofulvin
– Hair follicles: Arsenic, Mercury, Iodides
– Sweat: urea derivatives, amines, heavy metals Benzoic acid, Salicylic acid, Alcohol.
• Sweat patch Amphetamine, Ethanol, Heroin, cannabis, Nicotine
Gastrointestinal excretion (faecal elimination)
• Water soluble and ionised form of weakly acidic and basic drugs is excreted in GIT.
E.g. Nicotine, quinine, MgSO4, Streptomycin, Neomycin, Bacitracin, Cholestyramine, Erythropoitein, Chlorpromazine, Corticosteroids
Also, Visit: Packaging Materials in Pharmaceutical Industry