Acid Base Titration

Acid Base Titration
Titration is a method of analysis that will allow you to determine the precise endpoint of a reaction and therefore the precise quantity of reactant in the titration flask.
The chemical reaction involved in acid-base titration is known as neutralisation reaction.
Indicator
An indicator is a substance which is used to determine the end point in a titration.
In acid base titrations, organic substances (weak acids or weak bases) are generally used as indicators. They change their colour within a certain pH range.
The colour change and the pH range of some common indicators are tabulated below
|
Indicator |
pH range |
Colour change |
|
Methyl orange |
3.2–4.5 |
Pink to yellow |
|
Methyl red |
4.4–6.5 |
Red |
|
Litmus |
5.5–7.5 |
Red |
|
Phenol red |
6.8–8.4 |
Yellow to red |
|
Phenolphthalein |
8.3-10.5 |
Colourless to pink |
Theory of Indicator
An acid-base indicator is a weak acid or a weak base. Examples of indictors used in acid base reactions
Litmus
Phenolphthalein
Methyl orange
thymol blue, methyl yellow, methyl orange, bromphenol blue, bromcresol green, methyl red, bromthymol blue, phenol red, neutral red, phenolphthalein, thymolphthalein, alizarin yellow, tropeolin O, nitramine, and trinitrobenzoic acid.
|
Indicators |
pH range |
Color for weeak acid |
Color for conjugated base |
|
Metyl orange |
4–6 |
Orange |
Yellow |
|
Bromophenol |
6–7 |
Yellow |
Blue |
|
Thymol blue |
8–9 |
Yellow |
Blue |
|
Phenolphthalein |
9–10 |
Colourless |
Pink |
|
Alizarin yellow |
10–12 |
Yellow |
Red |
Theory of acid-base indicators:
Two theories have been proposed to explain the change of colour of acid-base indicators with change in pH.
Ostwald’s theory:
According to this theory, the colour change is due to ionisation of the acid-base indicator.
The unionised form has different colour than the ionised form.
The ionisation of the indicator is largely affected in acids and bases as it is either a weak acid or a weak base.
If the indicator is a weak acid, its ionisation is very much low in acids due to common H+ ions while it is fairly ionised in alkalise.
if the indicator is a weak base, its ionisation is large in acids and low in alkalises due to common OH- ions.
Considering two important indicators phenolphthalein (a weak acid) and methyl orange (a weak base), Ostwald theory can be illustrated as follows:
Phenolphthalein:
It can be represented as HPh.
It ionises in solution to a small extent as:
HPh ↔ H+ + Ph-
Colourless Pink Applying law of mass action,
K = [H+][Ph- ]/[HpH]
The un-dissociated molecules of phenolphthalein are colourless while Ph- ions are pink in colour.
Let us derive Handerson equation for an indicator
HIn + H2O ↔ H3O+ + In-
Acid form’ ‘Base form’ Methyl orange:
It is a very weak base and can be represented as MeOH. It is ionized in solution to give Me+ and OH- ions.
MeOH ↔ Me+ + OH
Yellow Red Applying law of mass action,
K = [Me+ ][OH- ]/[MeOH]
In presence of an acid, OH- ions are removed in the form of water molecules and the above equilibrium shifts to right hand side.
Thus, sufficient Me+ ions are produced which impart red colour to the solution.
On addition of alkali, the concentration of OH” ions increases in the solution and the equilibrium shifts to left hand side, i.e., the ionisation of MeOH is practically negligible.
Thus, the solution acquires the colour of unionised methyl orange molecules, i.e., yellow.
Indictors pKind pH
|
Methylorange |
3.7 |
3.1–4.4 |
|
Phenophthaline |
9.3 |
8.3–10.0 |
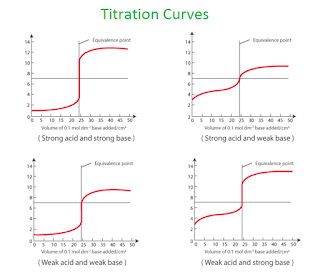
Titration curve
1) Titration of a strong acid with a strong base
In a strong acid–strong base titration, the acid and base will react to form a neutral solution. At the equivalence point of the reaction, hydronium (H+) and hydroxide (OH-) ions will react to form water, leading to a pH of 7
2) Titration of a weak acid with a strong base
In the titration of a weak acid with a strong base, the conjugate base of the weak acid will make the pH at the equivalence point greater than 7. Therefore, you would want an indicator to change in that pH range.
3) Titration of a strong acid with a weak base
In a weak base-strong acid titration, the acid and base will react to form an acidic solution.
A conjugate acid will be produced during the titration, which then reacts with water to
form hydronium ions.
This results in a solution with a pH lower than 7
4) Titration of a weak base with a weak acid
When a weak acid reacts with a weak base, the equivalence point solution will be basic if
the base is stronger and acidic if the acid is stronger; if both are of equal strength, then the
equivalence pH will be neutral.
Non-aqueous titration
Non-aqueous titration is the titration of substances dissolved in solvent other than water.
It is the most common titrimetric procedure used in pharmacopoeial assays and serves a double
purpose: it is suitable for the titration of very weak acid and very weak base, and it provides a solvent in which oirganic compound are soluble. The most commonly used procedure is the titration of organic base with perchloric acid in anhydrous acetic acid.
These assays sometimes take some perfecting in terms of being able to judge the endpoint precisely.
The Karl Fischer Titration for water content is another nonaqueous titration, usually done in methanol or sometimes in ethanol.
Since water is the analyte in this method, it cannot also be used as the solvent.
Need of Non aqueous titrations
Often times we need to perform an acid-base titration in non- aqueous solvent due to:
The analyte is too weak acid or a base to be titrated in H2O
Reactants or products are insoluble in H2O
Reactants or products react with H2O
Titration in H2O doesn’t allow a sharp end point but in a nonaqueous solvent with a stronger base than OH- it is possible to get an sharp end point
Bronsted Lowry; a general definition applicable to both aqueous and non-aqueousS systems
Lewis theory: Acids: electron pair acceptors
Bases: electron pair donors
Strong acids in water:
HCl + H2O → H3O+ + Cl–
(Acid) (Base) (Conjugated Acid) (Conjugated base)
Weak acids in water:
HCOOH + H2O <––––––––––-> H3O+ + HCOO–
(Acid) (Base) (Conjugated Acid) (Conjugated base)
Weak acids in non–aqueous solvents:
HCOOH + CH3NH2 <––––––-> CH3NH4+ + HCOO
(Acid) (Base) (Conjugated Acid) (Conjugatedbase)
It follows from these definitions that an acid may be either:
* an electrically neutral molecule, e.g. HCl, or
* a positively charged cation, e.g. C6H5NH3+,
or
* a negatively charged anion, e.g. HSO4-. A base may be either:
* an electricially neutral molecule, e.g. C6H5NH2, or an anion, e.g. Cl–.
* Substances which are potentially acidic can function as acids only in the presence of a base to which they can donate a proton. Conversely basic properties do not become apparent unless an acid also is present.
* The apparent strength of an acid or base is determined by the extent of its reaction with a solvent.
* In aqueous solution all strong acids appear equally strong because they react with the solvent to undergo almost complete conversion to hydronium ion (H3O+) and the acid anion.
* In a weakly protophilic solvent such as acetic acid, the extent of formation of the acetonium ion (CH3COOH2+) due to the addition of a proton provides a more sensitive differentiation of the strength of acids and shows that the order of decreasing strength for acids is perchloric, hydrobromic, sulfuric, hydrochloric, and nitric.
* Acetic acid reacts incompletely with water to form hydronium ion and is, therefore, a weak acid.
* In contrast, it dissolves in a base such as ethylenediamine, and reacts so completely with the solvent that it behaves as a strong acid. This so-called levelling effect.
Levelling effect or solvent levelling
Levelling effect or solvent: leveling refers to the effect of solvent on the properties of acids and bases.
The strength of a strong acid is limited (“leveled“) by the basicity of the solvent. Similarly the strength of a strong base is leveled by the acidity of the solvent.
When a strong acid is dissolved in water, it reacts with it to form hydronium ion (H3O+).[2] An example of this would be the following reaction, where “HA” is the strong acid:
HA + H2O → A− + H3O+
Any acid that is stronger than H3O+ reacts with H2O to form H3O+. Therefore, no acid stronger than H3O+ exists in H2O.
Similarly, when ammonia is the solvent, the strongest acid is ammonium (NH4+), thus HCl and a super acid exert the same acidifying effect.
The same argument applies to bases. In water, OH− is the strongest base. Thus, even though sodium amide (NaNH2) is an exceptional base (pKa of NH3 ~ 33), in water it is only as good as sodium hydroxide.
On the other hand, NaNH2 is a far more basic reagent in ammonia than is NaOH.
Solvents used in non-aqueous titration
Solvents that are used in non-aqueous titration are called non-aqueous solvents.
They are the following types:–
1. Aprotic Solvent
2. Protogenic Solvent
3. Protophillic Solvent
4. Amphiprotic Solvent
Aprotic solvents are neutral, chemically inert substances such as benzene and chloroform. They have a low dielectric constant, do not react with either acids or bases and therefore do not favor ionization.
The fact that picric acid gives a colorless solution in benzene which becomes yellow on adding aniline shows that picric acid is not dissociated in benzene solution and also that in the presence of the base aniline it functions as an acid, the development of yellow color being due to formation of the picrate ion.
Carbon tetrachloride and toluene come in this group; they possess low dielectric constants, do not cause ionization in solutes and do not undergo reactions with acids and bases.
Aprotic solvents are frequently used to dilute reaction mixture
Protogenic solvents are acidic substances, e.g. sulfuric acid. They exert a leveling effect on bases.
Anhydrous acids such as hydrogen fluoride and sulphuric acid fall in this category, because of their strength and ability to donate protons, they enhance the strength of weak bases.
Ex:- sulphuric acid , formic acid, propanoic acid, acetic anhydride etc.They have high dielectric constant and ionised because of their strength and ability to donate protons.
Protophilic solvents are the substances that possess a high affinity for protons. The over all reaction can be represented as:
HB+S ↔ SH+ + B-
The equilibrium in this reversible reaction will be generally influenced by the nature of the acid and the solvent.
Weak acids are normally used in the presence of strongly protophilic solvents as their acidic strengths are then enhanced and then become comparable to these of strong acids; this is known as the levelling effect.
Amphiprotic solvents have both protophilic and protogenic properties. Examples are acetic acid and the alcohols. They are dissociated to a slight extent.
The dissociation of acetic acid, which is frequently used as a solvent for titration of basic substances, is shown in the equation below:
CH3COOH ⇌ H+ + CH3COO−
Here the acetic acid is functioning as an acid. If a very strong acid such as perchloric acid is dissolved in acetic acid, the latter can function as a base and combine with protons donated by the perchloric acid to form protonated acetic acid, an onium ion:
HClO4 ⇌ H+ + ClO4−
CH3COOH + H+ ⇌ CH3COOH2+ (onium ion)
Since the CH3COOH2+ ion readily donates its proton to a base, a solution of perchloric acid in glacial acetic acid functions as a strongly acidic solution.
Titrants used in non aqueus titration
Acidic titrants:
o Perchloric acid
o p- Toluenesulfonic acid,
o 2,4-Dinitrobenzenesulfonic acid
Basic titrants
o Tetrabutylammonium hydroxide
o Sodium acetate
o Potassium methoxide
o Sodium aminoethoxide
Also, Visit: B. Pharmacy Notes | B. Pharma Notes | Study material Bachelor of Pharmacy