Determination of moisture/Water content by Karl Fischer Method

Determination of moisture content
Moisture or water content determination is a crucial analytical process in various industries, including pharmaceuticals, food, petrochemicals, and more. Accurate measurement of moisture content is vital as it impacts product quality and shelf life. One of the most precise methods for determining moisture is the Karl Fischer Method, a widely used titration technique. In this article, we’ll delve into the details of the Karl Fischer Method, its principles, applications, and much more.
What is Karl Fischer Method?
Karl Fischer Method, often abbreviated as KF Method, is a highly accurate and precise technique for measuring the moisture or water content in a sample. It was invented by the German chemist Karl Fischer in the 1930s. This method is based on a redox reaction that occurs when iodine is consumed by water in the sample. The amount of iodine consumed is directly proportional to the water content, making it an excellent choice for moisture determination.
Principles of Karl Fischer Titration
KF titration relies on the reaction between iodine and sulfur dioxide, which produces iodine ions. These iodine ions then react with water in the sample, forming hydroxide ions. The hydroxide ions react with sulfur dioxide, regenerating iodine, which can be precisely measured. The amount of iodine used in this process is directly proportional to the water content in the sample.
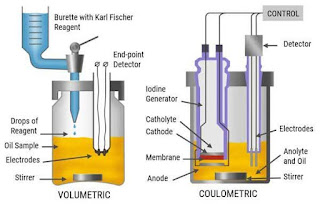
Types of Karl Fischer Reagents
There are two main types of Karl Fischer reagents: volumetric and coulometric. Volumetric titration uses a known volume of reagent to determine the water content, while coulometric titration uses a constant current to generate iodine, offering even higher precision.
Instruments Used in Karl Fischer Titration
To perform Karl Fischer titration, specialized instruments are required. These typically include a titration vessel, a titrator, a burette, and a stirrer. Modern titrators are equipped with computer interfaces for accurate data recording and analysis.
Equipment : Karl Fischer Titrator
Reagents : 1. AR grade Methanol
2. Pyridine free Karl Fischer Reagent
Sample Preparation
Proper sample preparation is crucial for accurate results. Samples must be ground, dried, and stored in a moisture-free environment to prevent contamination.
The Titration Process
During titration, the sample is titrated with a Karl Fischer reagent, and the endpoint is determined by a potentiometric method. The volume of reagent consumed is used to calculate the water content in the sample.
Procedure:
KF Factor Determination: Put methanol in the titration beaker enough to immerse the tip of platinum electrode.
Start stirrer and press start switch
Neutralize the methanol by titrating with KF-reagent.
Red indicating bulb glows at the end point.
Add one drop of water from the dropper of a previously weighed vial containing distilled water and reweigh to note the weight of water added.
Start titration by pressing the green start switch.
Note the volume of KF reagent required to neutralized the water (V)
Calculations in Karl Fischer Titration
The calculation involves stoichiometric equations that relate the volume of Karl Fischer reagent to the water content. These calculations are typically automated in modern titrators.
KF Factor = W/ V = mg/ml
Where:
W= Weight of water added.
V= Volume of KF reagent used.
Factors Affecting Karl Fischer Titration
Several factors can affect the accuracy of Karl Fischer titration, including the presence of interfering substances, temperature, and atmospheric moisture. Understanding and controlling these factors is essential for precise results.
Applications of Karl Fischer Titration
KF titration finds applications in a wide range of industries, including pharmaceuticals (determining drug purity), food (monitoring moisture content in food products), petrochemicals (measuring water in oils and fuels), and more.
Advantages and Disadvantages
The Karl Fischer Method’s primary advantages include high accuracy, precision, and the ability to measure low moisture levels. However, it may be time-consuming and requires specialized equipment.
Importance of Moisture Determination
Moisture content is critical in various industries, affecting product quality, stability, and safety. Accurate moisture determination ensures compliance with quality standards and regulatory requirements.
Karl Fischer Method vs. Other Methods
While the Karl Fischer Method is highly accurate, there are other techniques for moisture determination, such as loss on drying (LOD) and infrared spectroscopy. Each method has its advantages and limitations.
Safety Precautions
Handling reagents and chemicals in Karl Fischer titration requires safety precautions. Proper lab attire and knowledge of the materials used are essential to prevent accidents.
Conclusion
The Karl Fischer Method is a highly reliable and accurate technique for determining moisture content in various samples. Its applications are diverse, ranging from pharmaceuticals to petrochemicals. By understanding its principles, advantages, and limitations, industries can make informed decisions regarding moisture control and product quality.
FAQs
- Is the Karl Fischer Method the most accurate way to measure moisture content? The Karl Fischer Method is known for its high accuracy and precision, making it one of the best methods for moisture determination.
- What are the typical industries that use Karl Fischer titration? Industries such as pharmaceuticals, food, petrochemicals, and cosmetics rely on Karl Fischer titration for moisture analysis.
- Can the Karl Fischer Method be used for both high and low moisture samples? Yes, the method is versatile and can accurately determine moisture content in a wide range of samples, from very dry to highly humid.
- Are there any safety concerns when using the Karl Fischer Method? Yes, handling reagents and chemicals in Karl Fischer titration requires safety precautions. It’s essential to follow safety guidelines to prevent accidents.
- How does the Karl Fischer Method compare to other moisture determination techniques? While the Karl Fischer Method is highly accurate, other methods like loss on drying and infrared spectroscopy also have their applications. The choice depends on specific needs and sample types.
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf