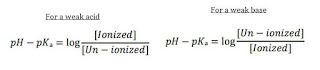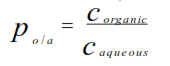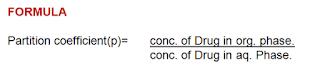Preformulation Studies

Preformulation Studies
Pre-formulation
It is defined as the phase of research and development in which preformulation studies characterize physical and chemical properties of a drug molecule in order to develop safe, effective and stable dosage form.
Preformulation testing is the first step in the rational development of dosage forms.
It can be defined as an investigation of physical and chemical property of a drug substance alone and when combined with excipients.
NEED of Pre-Formulation Study
Preliminary evaluation
Molecular optimisation
Suitability of Excipients
Suitability of dosage foam
Objective of Preformulation Studies
To establish the physico-chemical parameters of a new drug entity
To determine its kinetics and stability
To establish its compatibility with common excipients
It provides insights into how drug products should be processed and stored to ensure their quality
Major Area of Preformulation Research
➢ ORGANOLEPTIC CHARACTERS
➢ BULK CHARACTERS
❑ Crystallinty and polymorphism
❑ Hygroscopicity
❑ Fine particle characterization
❑ Powder flow properties
➢ SOLUBILITY ANALYSIS
❑ ionization constant-PKa
❑ pH solubility profile
❑ Common ion effect-Ksp
❑ Thermal effects
❑ Solubilization
❑ Partition co-efficient
❑ Dissolution
➢ STABILITY ANALYSIS
❑ Stability in toxicology formulations
❑ Solution stability
❑ pH rate profile
❑ Solid state stability
❑ Bulk stability
❑ Compatibility
ORGANOLEPTIC CHARACTERS
❖ Colour,odour, taste of the new drug must be recorded
COLOUR ODOUR TASTE
❑Off-white ❑ pungent ❑ Acidic
❑Cream yellow ❑ sulphurous ❑ Bitter
❑tan ❑ Fruity ❑ Bland
❑shiny ❑ Aromatic ❑ Intense
❑ Odourless ❑ Sweet
❑ Tasteless
Preformulation Bulk Characterization
Crystallinity
Crystal habit & internal structure of drug can affect bulk & physicochemical property of molecule.
Crystal habit is description of outer appearance of crystal.
Internal structure is molecular arrangement within the solid.
Change with internal structure usually alters crystal habit.
Eg. Conversion of sodium salt to its free acid form produce both change in internal structure & crystal habit.
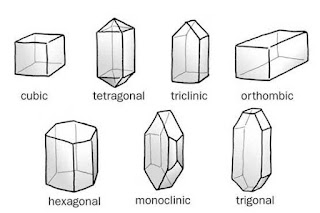
Different shapes of crystals
Depending on internal structure compounds is classified as
1. Crystalline
2. Amorphous
Crystalline compounds are characterized by repetitious spacing of constituent atom or molecule in three dimensional array.
In amorphous form atom or molecule are randomly placed.
Solubility & dissolution rate are greater for amorphous form than crystalline, as amorphous form has higher thermodynamic energy.
Eg. Amorphous form of Novobiocin is well absorbed whereas crystalline form results in poor absorption.
Polymorphism
It is the ability of the compound to crystallize as more than one distinct crystalline species with different internal lattice.
Different crystalline forms are called polymorphs.
Polymorphs are of 2 types
1. Enatiotropic
2. Monotropic
The polymorph which can be changed from one form into another by varying temp or pressure is called as Enantiotropic polymorph.
Eg. Sulphur.
One polymorph which is unstable at all temp. & pressure is called as Monotropic polymorph.
Eg. Glyceryl stearate.
Polymorphs differ from each other with respect to their physical property such as
• Solubility
• Melting point
• Density
• Hardness
• Compression characteristic
Eg. 1)Chloromphenicol exist in A,B & C forms, of these B form is more stable & most preferable.
ANALYTICAL METHODS FOR THE CHARACTERIZATION OF SOLID FORMS
- Microscopy
- Hot stage microscopy
- Thermal analysis
- X-ray diffraction
- Infrared (IR) spectroscopy
- Proton magnetic resonance (PMR)
- Nuclear magnetic resonance (NMR)
- Scanning electron microscopy (SEM)
Microscopy
Material with more than one refractive index are anisotropic & appear bright with brilliant colors against black polarized background.
The color intensity depends upon crystal thickness.
Isotropic material have single refractive index and this substance do not transmit light with crossed polarizing filter and appears black.
Advantage :
By this method, we can study crystal morphology & difference between polymorphic form.
Disadvantage :
This require a well trained optical crystallographer, as there are many possible crystal habit & their appearance at different orientation.
Hot stage microscopy
The polarizing microscope fitted with hot stage is useful for investigating polymorphism, melting point & transition temp.
Disadvantage :
In this technique, the molecules can degrade during the melting process.
Thermal analysis
Differential scanning calorimetry (DSC) & Differential thermal analysis are (DTA) are particularly useful in the investigation of polymorphism.
It measures the heat loss or gain resulting from physical or chemical changes within a sample as a function of temp.
For characterizing crystal forms , the heat of fusion can be obtained from the area under DSC- curve for melting endotherms.
Similarly, heat of transition from one polymorph to another may be calculated.
A sharp symmetric melting endotherm can indicate relative purity of molecule.
A broad asymmetric curve indicates presence of impurities.
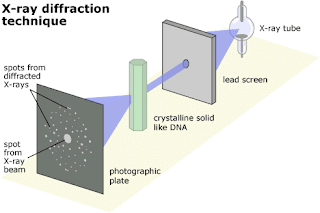
X-ray diffraction
Working :
When beam of nonhomogenous X-ray is allow to pass through the crystal, X-ray beam is diffracted & it is recorded by means of photographic plate.
Diffraction is due to crystal which acts as 3 dimensional diffraction grating toward X-ray.
Random orientation of crystal lattice in the powder causes the X-ray to scatter in a reproducible pattern of peak intensities.
The diffraction pattern is characteristic of a specific crystalline lattice for a given compound.
An amorphous form does not produce a pattern mixture of different crystalline forms.
Single – Crystal x-ray provide the most complete information about the solid state.
HYGROSCOPICITY
Many drug substances, particularly water –soluble salt forms, have a tendency to adsorb atmospheric moisture.
Adsorption and moisture content depend upon the atmospheric humidity, temperature, surface area, exposure and the mechanism of moisture uptake.
The degree of Hygroscopicity is classified into four classes:
- Slightly hygroscopic: increase in weight is ≥ 0.2% w/w and < 2% w/w
- Hygroscopic : increase in weight is ≥ 0.2 % w/w and < 15 % w/w
- Very hygroscopic : increase in weight is ≥ 15% w/w
- Deliquescent : sufficient water is adsorbed to form a solution
Hygroscopicity is tested by:
- Samples are exposed to the moisture
- exposed to controlled relative humidity environments
- moisture uptake is monitored at different time points
- Gravimetry
- Karl Fischer Titration
- Gas chromatography
PARTICLE SIZE
Particle size is characterized using these terms :
Very coarse, Coarse, Moderately coarse, Fine ,Very fine .
Particle size can influence variety of important factors :
- Dissolution rate
- Suspendability
- Uniform distribution
- Penetrability
- Lack of grittiness
Methods to Determine Particle Size
- Sieving (5µ-150µ)
- Microscopy(0.2µ-100µ)
- Sedimentation rate method(1µ-200µ)
- Light energy diffraction(0.5µ-500µ)
- Laser holography(1.4µ-100µ)
POWDER FLOW PROPERTIES
➢ Powder flow properties can be affected by change in particle size, shape & density.
➢ The flow properties depends upon following-
1. Force of friction.
2. Cohesion between one particle to another.
➢ Fine particle posses poor flow by filling void spaces between larger particles causing packing & densification of particles.
➢ By using glident we can alter the flow properties. e.g. Talc
Determination of Powder Flow Properties
➢ By determining Angle of Repose.
➢ A greater angle of repose indicate poor flow.
➢ It should be less than 30°. & can be determined by following equation.
tan θ = h/r.
where, θ = angle of repose. h=height of pile. r= radius.
Angle of Repose ( In degree) Type of Flow
<25 Excellent
25-30 Good
30-40 Passable
>40 Very poor
Methods to determine angle of repose
➢ Static angle of repose
- Fixed-funnel method
- Fixed-cone method
➢ Kinetic or dynamic method
- Rotating cylinder method
- Tilting box method
Determination of Powder Flow Properties
➢ Measurement of free flowing powder by compressibility.
➢ Also knaown as Carr’s index.
CARR’S INDEX(%) =(TAPPED DENSITY – POURED DENSITY) X 100
TAPPED DENSITY
➢ It is simple, fast & popular method of predicting powder flow characteristics.
Determination of Powder Flow Properties
Carr’s Index Type of flow
5-15 Excellent
12-16 Good
18-21 Fair To Passable
23-35 Poor
33-38 Very Poor
>40 Extremely Poor
SOLUBILITY STUDIES
1. Solution phase equilibrium with solid phase at a stated temperature and pressure.
2. Determines amount of drug dissolved , amount of drug available for absorption.
3. Solubility reduction is carried out in certain conditions:
❖ Enhancement of chemical stability.
❖ taste masking products.
❖ Production of sustained release products.
Descriptive term Parts of solvent required for 1 part of solute
Very soluble Less than 1
Freely soluble From 1 to 10
Soluble From 10 to 30
Sparingly soluble From 30 to 100
Very slightly soluble From 100 to 1000
Practically insoluble From 1000 to 10,000
Slightly soluble 10,000 and over
The equilibrium solubility is based on the phase-solubility technique proposed by Higuchi-Connors .
Method
- Drug dispersed in solvent in a closed container
- agitated at a constant temperature using shakers
- samples of the slurry are withdrawn as a function of time
- clarified by centrifugation and assayed by HPLC, UV, GC etc
pKa determination
pKa is the dissociation constant of a drug
The un-ionized drug is lipid soluble thus permeates through lipid membrane.
The ionized substance is lipid insoluble therefore permeation is slow
Degree of ionization depends on pH
Determined by uv spectroscopy, potentiometric titration, titrimetric method
SOLUBILIZATION
“Solubilization is defined as the spontaneous passage of poorly water soluble solute molecules into an aqueous solution of asoap or detergent in which a thermodynamically stable solution is formed”.
➢It is the process by which apparent solubility of an otherwise sparingly soluble substance is increased by the presence of surfactant micelles .
❑ MICELLES: –
➢ The mechanism involves the property of surface active agents to form colloidal aggregates known as micelles.
➢ When surfactants are added to the liquid at low concentration they tend to orient at the air-liquid interface .
➢ On further addition of surfactant the interface becomes completely occupied and excess molecules are forced into the bulk of liquid.
➢At very high concentration surfactant molecules in the bulk of liquid begin to form micelles and this concentration is know as CRITICAL MICELLE CONCENTRATION (CMC)
General Method of Increasing the Solubility
➢ Addition of co-solvent
➢ pH change method
➢ Reduction of particle size
➢ Temperature change method
➢ Hydotrophy
➢ Addition of Surfactant
➢ Dielectrical Constant
➢ Complexation
Partition Coefficient
A measurement of drug lipophilicity i,e the ability to cross the cell membrane
Distribution coefficient
The octanol-water system is widely accepted to explain these phenomenon.
Buccal membrane : butanol-pentanol system
Blood-Brain barrier: chloroform-cyclohexane
Determined by SHAKE FLASK METHOD
SHAKE FLASK METHOD
Drug is shaken between octanol and water.
Aliquot is taken and analyzed for drug content
RULE OF FIVE : for drug permeates through passive diffusion
1. Log P is greater than 5
2. Molecular weight >500
3. There are more than 5 hydrogen bond donors (number of NH + OH)
4. There are more than 10 hydrogen bond acceptors (number of hydrogen + oxygen )
5. Molar refractivity should be between 40-130
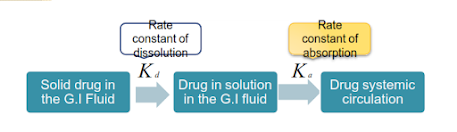
DISSOLUTION
When Kd << Ka ,dissolution is significantly slower and the absorption is described as dissolution-rate limited.
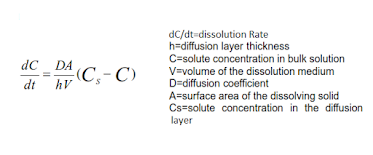
The dissolution rate of drug substance in which surface area is constant during dissolution is described by Noyes-Whitney equation.
Constant surface area is obtained by compressing powder into a disc of known area with a die and punch apparatus.
Hydrodynamic conditions are maintained with Static-disc dissolution apparatus and Rotating disc apparatus
static dissolution apparatus and rotating disc apparatus
STABILITY ANALYSIS
1. Solution stability
2. Solid state stability
1. SOLUTION STABILITY
▪ The decomposition of drug occurs through hydrolysis, oxidation, photolysis.
➢ Hydrolysis (anaesthetics, vitamins etc )
a) Ester hydrolysis
R’-COOR + H+ + OH- ———-> RCOOH + ROH
ester acid alcohol
b) Amide hydrolysis
RCONHR’ + H+ + OH- ————-> RCOOH + H2N-R’
Amide Acid Amine
➢ Oxidation
used to evaluate the stability of pharmaceutical preparations
Eg : steroids, vitamins, antibiotics, epinephrine
Autoxidation
Materials + molecular oxygen
homolytic fission
Free radicals are produced.
Oxygen sensitivity is measured by bubbling air through the compound or adding hydrogen peroxide.
➢ Photolysis
pharmaceutical compounds
exposure to uv light
absorbs the radiant energy
undergoes degradative reactions
2. SOLID-STATE STABILITY
1o objective: identification of stable storage conditions.
identification of compatible excipients
Solid-state stability depends on the temperature , light, humidity, polymorphic changes, oxidation.
Solid-State Stability profile of a new compound
Samples are placed in open vials and are exposed directly to a variety of temperatures, humidities, and light intensities for up to 12 weeks.
Vials exposed to oxygen and nitrogen to study the surface oxidation and chemical stability , polymorphic changes and discolouration.
Stability data obtained at various humidities may be linearized with respect to moisture using the following apparent decay rate constant (KH )
k H = [ gpl].k 0
gpl= concentration of water in atmosphere in units of grams of water per liter of dry air .
ko = decay rate constant at zero relative humidity
Mole fraction of the solid that has liquefied (Fm ) is directly proportional to its decay rate.
Drug- excipient compatibility
Compatibility test play a very important role in the preformulation studies of oral dosage forms
An incompatibility in the dosage form can result in any of the following changes:
➢ Changes in organoleptic properties
➢ Changes in dissolution performance
➢ Physical form conversion
➢ An decrease in potency
METHOD
- Drug + Excipients (1:1)
- Powder samples dispersed into glass ampoules
- 1 ampoule or 1 ampoule (sample + water)
- stored at a particular temperature (500 C) and analysed
In emulsions the studies include measuring the critical micelle concentration of the formulations
For oral use preparations compatibility of the ingredients (ethanol, glycerine, syrup, sucrose, buffers and preservatives)
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf