EXTRACTION AND ISOLATION OF ALKALOIDS

EXTRACTION AND ISOLATION OF ALKALOIDS
The extraction of alkaloids is based on their basic character and solubility pattern.
Extraction is usually served by one of the following general methods;
1. The plants are defatted with petroleum ether, especially in case of seeds and leaves to remove the fat-soluble constituents and then with polar solvents.
The extract is concentrated under reduced pressure and treated with alkali so that the free bases convert into their salts and are separated with organic solvents. T
his process is known as the Stas-Otto process.
This method is frequently used in the extraction of ergotamine from ergot.
2. The powdered material is moistened with water and mixed with lime, which combines with acids, tannins, and other phenolic substances and sets free the alkaloid salts.
Extraction is then carried out with organic solvents such as ether or petroleum spirit.
The concentrated organic liquid is then shaken with aqueous acid and allowed to separate.
Alkaloid salts are now in an aqueous liquid, while many impurities remain behind in the organic liquid.
3. The powdered material is extracted with polar solvents such as water or aqueous alcohol containing dilute acid.
Pigments and other unwanted materials are removed by shaking with chloroform or other organic solvents.
The free alkaloids are then precipitated by the addition of excess sodium bicarbonate or ammonia and then separated by filtration or by extraction with organic solvents.
4. The extract is treated with ammonia to convert the alkaloid salts into their free bases.
Such liberated alkaloids in the free base form are conveniently extracted with organic solvents like ether, benzene, chloroform etc.
This method is not useful for the isolation of alkaloids of quaternary nitrogen.
5. The alkaloids present in the extract are converted into their reineckates by treating with H[Cr(NH3)2(SCN)4] (Reinecke‘s solution).
The product is then dissolves in acetone and then passed this solution through an ion exchange column which afforded the alkaloids in a high state of purity.
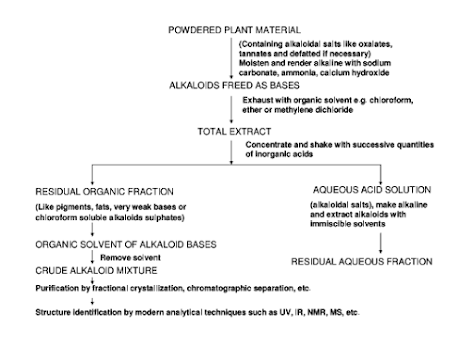
General scheme for extraction of alkaloids
Further purification of crude extract of alkaloids is done by following ways, which may, however, vary for individual alkaloids.
Direct crystallization from solvent: It is a simple method of isolation in which the alkaloids crystallise directly by fractionation process and may not be useful in case of complex mixtures.
Steam distillation: This method is specially employed for volatile liquid alkaloids like coniine, sparteine and nicotine.
However this method is not suitable for alkaloids of high molecular weights.
In this method, the aqueous extract is made alkaline with caustic soda or sodium carbonate and then alkaloids are distilled off in steam.
Gradient pH technique: There is variation in the extent of basicity of various alkaloids of same plant.
Based on this property, the crude alkaloid mixture is dissolved in 2% tartaric acid solution and extracted with benzene so that the first fraction contains neutral and/or very weakly basic alkaloids.
The pH value of the aqueous solution is increased gradually by 0.5 increments to pH 9 and extraction is carried out at each pH with organic solvents.
By this way, the alkaloids of different basicity are extracted and strongly basic alkaloids extracted at the end.
Chromatographic techniques: Chromatography is an ideal method for separation of a vast number of alkaloids.
The separation of alkaloids carried out by using stationary and mobile phase of different organic solvents.
The different techniques of chromatography used for the separation of individual alkaloids from the complex mixture are as following;
i. Paper chromatography (PC): This technique is simplest and most widely used among other chromatographic techniques because of its applicability to isolation, identification and some times quantitative determination of all type of natural products.
It is a partition as well as an absorption type technique, in which the mobile phase is either individual or mixture of organic solvents and the stationary phase is hydrophilic surface of paper.
The choice of the solvent used to run a chromatogram depends upon the nature of alkaloid.
ii. Thin layer chromatography (TLC): TLC is an important tool extensively used for identification, separation and determination of the purity of isolated alkaloid.
Although TLC method is used in qualitative analysis but it having a great importance in quantitative analysis also.
The TLC separation of alkaloids can be performed on silica gel, alumina, cellulose powder or kieselguhr.
Silica gel is the most active stationary phase and good separation is achieved even when several milligrams substance applied.
This method is also used for preparative separation of alkaloids.
Extraction and Isolation of Alkaloids FAQs
- Are all alkaloids derived from plants? Alkaloids are primarily found in plants, but some synthetic alkaloids exist.
- How do extraction methods affect the potency of isolated alkaloids? Different extraction methods can impact the potency of isolated alkaloids, requiring careful selection based on the desired outcome.
- What role do alkaloids play in traditional medicine? Alkaloids in traditional medicine often serve as remedies for various ailments, showcasing the historical significance of these compounds.
- Are there any risks associated with the burstiness of isolation techniques? While burstiness introduces unpredictability, researchers implement controls to manage and optimize isolation outcomes.
- How can the extraction and isolation of alkaloids contribute to environmental sustainability? Sustainable practices, such as green extraction methods and ethical sourcing, play a crucial role in minimizing the environmental impact of alkaloid production.