MANUFACTURE OF DRUGS

MANUFACTURE OF DRUGS
Manufacture in relation to any drug or cosmetic, includes any process or part of process for making, altering, ornamenting, finishing, packing, labeling, braking up or otherwise treating any drug or cosmetic with a view to its sale & distribution but does not include the compounding or dispensing of any drug or packing of any drug in ordinary course of retail business
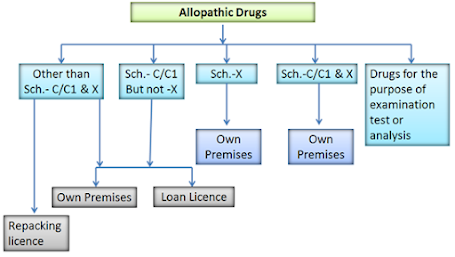
Following licenses are provided for manufacture of drugs under D&C Act
-
- Drugs other than those specified in Schedule C, C1 & X
- Drugs specified in Schedule C, C1 but not specified in Schedule X
- Drugs specified in Schedule C, & C1
- Drugs specific in Schedule X but not in Schedule C & C1
- Drugs specified in Schedule C, C1 and X
- Drugs for the purpose of examination, test or analysis
- Loan Licenses
- Repacking Licenses
- Blood products
Repacking is also a manufacturing for the purpose of the act.
If drugs are manufactured in more than one set of premises, a separate application is to be made & separate license shall be issued in respect of each such premises.
Licenses for manufacture or sale or distribution of drugs are granted or renewed by Central License Approving Authority (CLAA) appointed by the central government.
CLAA can delegate his power of signing licenses to any other person under his control with approval of the Central Government.
Manufacture
- Prohibition of manufacture
- Manufacture of other than in Schedule-C/C1
- Manufacture of those in Schedule-C/C1
- Manufacture of Schedule-X drugs
- Loan license
- Repackaging license
- Offences & Penalties
Types of manufacturing licenses
Prohibition of manufacture
- Drug not of standard quality or misbranded, adulterated or spurious.
- Patent or Proprietary medicine
- Drugs in Schedule-J
- Risky to human beings or animals
- Drugs without therapeutic value
- Preparation containing cyclamates
Prohibition for the manufacture & sale of Certain Drugs
- From the date notified by the State Government, no person shall himself manufacture for sale or distribution or sell or distribute-
ü Any drug which is not of standard quality or is misbranded, adulterated or spurious;
ü Any cosmetic which not of standard quality or is misbranded, adulterated or spurious;
ü Any patent or proprietary medicine whose formulae is not disclosed on label or the container;
ü Any drug which purports to cure, mitigate or prevent any disease specified in Schedule J;
ü Any cosmetic containing any ingredient which may render it unsafe or harmful for use;
ü Any drug or cosmetic in contravention of this act or rules thereunder;
ü Any drug or cosmetic which has been imported or manufactured in contravention of the provisions of this Act or Rules thereunder or in contravention of the conditions of a license.
- Every person not being manufacturer of a drug or cosmetic or his agent for the distribution shall if so required disclose to the inspector the name address and other particulars of the person from whom he procured the drug or cosmetic.
- A drug or cosmetic shall not be rendered to be misbranded, adulterated or spurious or below standard quality, if-
-There has been added thereto some innocuous substance or ingredient required for its manufacture or preparation as an article of commerce in state fit for carriage or consumption, & not to increase the bulk, or weight or measure of the drug or cosmetic or to conceal its inferior quality or other defect.
-In process of manufacture, preparation or conveyance some extraneous substance has been unavoidably become inter-mixed with it, however this does not apply in relation to any sale or distribution of the drug or cosmetic occurring after the vendor or distributor becomes aware of such inter-mixture.
- There are two types of conditions for all manufacturing licenses
-Conditions which are to be satisfied before a license is granted
-Conditions which are to be satisfied after the license is granted.
Manufacture of Drugs other than those specified in Schedule C & C1
- Application for the grand or renewal of license for the manufacture of drugs other than those specified in schedule C, c1 & X ‘d be made to LA in Form 24 & for manufacture of Schedule X drugs in Form 24F. Respective licenses are issued in form 25 & 25F
- Application for grand/renewal of such license shall be made for up to 10 items in each category in Form 24-A accompanied by fee of 6000 & an inspection fee of Rs. 1500 to LA & license shall be issued in Form 25A.
- Additional fee of Rs 300 per item is payable for each additional item
- License in form 25 or 25F remains valid for a period of 5 years on and from the date on which it is issued.
- If application for renewal is made before its expiry, or application made within 6 months of expiry, after payment of additional fee, the license shall continue to be valid
- License shall deemed to have expired if the application for its renewal is not made within 6 months of its expiry.
Conditions
- Premises should comply with schedule ‘M’
- Adequate facility for testing, separate from manufacturing
- Adequate storage facility
- Records maintained for at least 2 years from date of Exp.
- Should provide sample to authority
- Furnish data of stability
- Maintain the inspection book
- Maintain reference samples from each batch
Manufacture of drugs those in Schedule-C/C1 (Biological)
Conditions
- Drugs must be issued in previously sterilized sealed glass or suitable container
- Containers should comply with Schedule-F
- Some classes should be tested for aerobic & anaerobic micro-organism.eg. Sera, Insulin, Pituitary hormones.
- Serum should be tested for abnormal toxicity
- Parenteral in doses of 10 ml or more should be tested for freedom from Pyrogens
- Separate lab. for culture & manipulation of spore bearing Pathogens
- Test for sterility should be carried out.
Manufacture of drugs specified in Schedule C, C1 & X
- Application for the license of manufacturing drugs specified in Schedule C, C1 excluding those specified in Schedule X should be made to the LA in Form 27 & for manufacture of drugs specified in Schedule C, C1 & X in for 27B. Respective licenses are issued in Form 28 & 28B.
- Application for including any additional drug in the license should be accompanied by a fee of Rs.50 for each drug subject to a maximum of Rs.500
- Conditions for the grant of license: Before the grand of license, the following conditions must be complied by the applicant
1. The manufacture will be conducted under the active direction of a competent technical staff consisting at least one person who is a full time employee & who is
-A graduate in pharmacy/pharmaceutical chemistry of a recognized University with at least 18 months practical experience after graduation in manufacture of drugs to which this license applies.
-A graduate in science of a recognized University who passed in degree with chemistry or microbiology as principal subject & had al least 3 years experience in the manufacture of drugs to which the license applies.
C,C1 …27
C,C1,X……………………………27B
-A graduate in medicine of a recognized University with at least 3 years’ experience in manufacture of relevant drugs; or
-A graduate in chemical engineering of a recognized University with at least 3 years’ experience in manufacture of relevant drugs; or
-Holding any foreign qualification comparable in quality, content and training with above qualifications & is permitted to work as competent staff by Central Government
2. The factory conditions must comply with the conditions prescribed in Schedule M and M3
3. Applicant should provide adequate space, plant & equipment for any or all manufacturing operations as prescribed in Schedule M & M3
4. Applicant should provide adequate staff, premises and laboratory equipment for carrying out such tests for strength, quality & purity of substances as required under the rules.
5. Adequate facilities for the storage of manufactured drugs should be provided.
6. Data on stability of drugs that may deteriorate, for fixing the date of expiry shall be furnished to LA.
7. Licensee shall comply with requirements of GMP.
8. For manufacture of patent or proprietary medicines, data should be provided to LA that justifies that the medicines are: stable under conditions of recommended storage.
contains such ingredients & in such quantities for which there is therapeutic justification
- License in form 28 & 28B remains valid for a period of 5 years on and from the date on which it is issued.
- If application for renewal is made before its expiry, or application made within 6 months of expiry, after payment of additional fee, the license shall continue to be valid
- License shall deemed to have expired if the application for its renewal is not made within 6 months of its expiry.
- Large Volume Parenteral means the sterile solutions indented for parenteral administration with a volume of 100 ml or more in one container of the finished dosage form indented for single usage.
Conditions of the License
1. Licensee should provide & maintain, adequate staff & adequate premises and plant for the proper manufacture & storage of substances
2. Licensee should maintain records of the manufacture as per particulars given in schedule U.
3. Licensee should allow Inspectors to enter any premises where manufacture is carried on & to inspect the process of the manufacture.
4. Licensee should allow inspectors to inspect all registers and records maintained under these rules & to take samples of manufactured product
5. should allow the LA to inspect if any changes in expert staff & any material changes in premises or plant since date of last inspection.
6. On request by LA licensee should furnish form every batch, a sample of adequate quantity for any examination
7. If any batch has been found out by LA not to confirm with the standards, licensee should withdraw the remainder of batch from sale.
8. should maintain a Inspection book to enable inspector to record his impression.
9. should maintain reference samples of each batch of drugs manufactured by him, in a quantity twice than that sufficient for conducting all tests.
10. should forward to LA of state a statement of sales effected to manufacturers, wholesalers, retailers, hospitals, nursing homes, dispensaries every three months.
Manufacture of Schedule-X drugs
Conditions
§ Accounts of all transactions regarding manuf. should be maintained in serially.(Preserved for 5 years)
§ Have to sent invoice of sale to licensing authority every 3 months
§ Store drugs in direct custody of responsible person.
§ Preparation must be labeled with XRx
§ Marketed in packings not exceeding
§ 100 unit dose –Tablets/Capsules
§ 300 ml- Oral liquid
§ 5 ml – Injection
Manufacture of Drugs for Examination, Tests or Analysis
- License is necessary for the manufacture of any drug in small quantity for the purpose of examination, test or analysis.
- If a person proposing to manufacture does not hold license i) to manufacture drugs other than those specified in Schedule C, C1 & X, or ii) to manufacture drugs specified in Schedule C, C1 in respect to such drugs; he should obtain license in Form 29.
- If drug is not recognized as safe for use, license in Form 29 is only granted after producing no objection certificate from LA appointed by Central Government.
- License remains valid for a period of one year time
- Drugs should be kept in containers bearing labels indicating the purpose for which it has been manufactured.
- If the drugs are to be supplied, it should bear label stating name & address of manufacturer, scientific name of substance & purpose for which it has been manufactured.
Conditions for License
-
- Drugs should be used exclusively for the purpose for which they are manufactured
- Licensee should allow inspector to inspect the premises & satisfy himself that only examination, test or analysis is being conducted.
- Licensee should keep record of quantity of drugs manufactured and supplied to any person.
- Licensee should maintain inspection book to enable inspector to record his impression and defects noticed.
- Licensee must comply with any rules made subsequently and of which the LA has given him NLT one months’ notice.
Manufacture of New Drugs
- Defined as a drug the composition of which is such that it is not generally recognized among experts as safe for use under conditions recommended; or
- Suggested on the label & includes any drug the composition of which is such that the drug as a result of investigations for determining its safety for use under such conditions, is so recognized but which has not otherwise than during course of such investigations, been used to any large extend for any appreciable length of time under the said conditions
- Provisions applicable for the manufacture of new drugs whether classifiable under schedule C & C1 or otherwise:
-No new drug can be manufactured unless prior approval of the LA has been taken.
-Applicant should produce all documentary & other evidence relating to the standards of quality, purity, strength & such other information as may be required including the results of therapeutic trials carried out on the new drug.
-While applying for a license to manufacture a new drug, or its preparations an applicant should produce along with his application evidence that the drug has already been approved.
Loan License
Definition:
A person (applicant) who does not have his own arrangements (factory) for manufacture but who wish to manufacturing facilities owned by another licensee. Such licenses are called Loan licenses.
Licence is obtained from licensing authority (FDA) on application in prescribed forms (24-A, 27-A) with prescribed fees.
Loan licenses are issued for:
1) Drugs other than specified in C/C1 & X.
2) Drugs specified in Schedule-C/C1
- A loan license means a license which a LA may issue to a applicant who does not have his own arrangements for manufacture but who intends to avail himself of the manufacturing facilities owned by another licensee.
- Issued for the manufacture for sale or distribution of drugs other than those specified in Schedule C, C1 & X.
- Application for license is made in Form 24-A & the license is issued in Form 25-A.
- Before grant of license, the LA shall get the premises inspected by one or more inspectors.
- Inspector shall check into all the portions of the plant & shall also inquire in professional qualification for the technical staff employed.
- For the manufacturing of additional items, an application must be made to LA.
- Licensee is required to test each batch of raw materials & finished products & the records must be maintained for a period of 5 yrs from the date of manufacture. (2yrs in case of drugs having expiry date, from the date of expiry)
- Loan license is deemed to be cancelled or suspended if license owned by loan licensee, whose manufacturing facilities is been availed by licensee is cancelled or suspended.
Repacking Licenses
Repacking license are granted for breaking up of any drug other than those specified in Schedule C, & C1, on application to LA in Form 24B & license is issued in Form 25B subject to satisfying the following conditions:
- The repacking operation must be carried out under hygienic conditions & under supervision of competent staff namely,
a) A person who holds an approved Diploma in Pharmacy or is an Registered Pharmacist.
b) A person who has passed intermediate examination with Chemistry as principal subject.
c) A person who has passed matriculation & has at least 4 yrs. practical experience in manufacturing, dispensing or repacking of drugs.
- Factory conditions must specify conditions prescribed in Schedule M.
- Applicant must have in his premises adequate facilities for the testing of drugs. Which is separate from the repacking unit.
- License must be kept at licensed premises & produced on request of DI
- Any change in competent staff must be reported to LA
- For repacking of any additional items, application must be made to LA.
- The label on repacked drugs should mention the name & address of the licensee & his license number preceded by the word ‘Rpg. Lic. No.”
- The license remains valid up to 31st December of the year following the year in which it is grated.
Definition:
Process of breaking up any drug from a bulk container into small packages and labeling with a view to their sale and distribution.
Repackaging of drugs is granted of drugs other than Schedule-C/C1 and X.
Penalties Related to Manufacture
| OFFENCES | PENALTIES |
| Manufacture of any spurious drugs |
a) 1-3 years imprisonment and Rs.5000 fine b) 2-6 years imprisonment & Rs.10000 fine on subsequent conviction |
| Manufacture of adulterated drugs |
a) 1 year imprisonment & Rs.2000 fine b) 2 years imprisonment & Rs.2000 fine for subsequent conviction |
| Manuf. of drugs in contravention of the provisions |
a) Imprisonment up to 3 months & Rs.500 fine b) Imprisonment up to 6 months & Rs.1000 fine on subsequent conviction |
Manufacture of cosmetics
Prohibited for the following classes :
- Misbranded or spurious cosmetics and of substandard quality
- Cosmetics containing hexachlorophene or mercury compounds
- Cosmetics containing color which contain more than-
– 2 ppm of arsenic
– 20 ppm of lead
– 100 ppm of heavy metals
- Eye preparations containing coal-tar color
FAQs
- What is the primary purpose of Schedule X drugs?
- Schedule X drugs are primarily used to regulate psychotropic substances and narcotics to prevent their misuse and illegal distribution.
- What are loan licenses in drug manufacturing?
- Loan licenses are licenses granted to manufacturers who do not own their manufacturing facilities but wish to produce drugs.
- Why is quality control crucial in drug manufacturing?
- Quality control is vital to ensure that drugs are safe, effective, and meet the required standards, protecting public health.
- How do regulatory authorities oversee drug manufacturing?
- Regulatory authorities monitor drug manufacturing by inspecting facilities, reviewing documentation, and ensuring compliance with regulations.
- What are the key challenges in drug manufacturing?
- Common challenges include regulatory compliance, quality control, and ensuring consistent product quality. Manufacturers must also address issues like raw material sourcing and distribution.
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf