Flame Photometry

Objectives
By the end of this session, students will be able to:
• Explain the principle of flame photometry and instrumentation
• Outline the factors affecting the intensity of flame emission
Atomic Spectroscopy
To understand the relationship of these techniques to each other, It is important to understand the atom itself and the atomic process involved in each technique.
Practically, the ratio of the excited to ground state atoms is extremely small. Therefore, The absorption spectrum is usually only associated with transitions from the ground state to higher energy states
N1: No. of excited atoms
N°: No. of ground state atoms
ΔE: excitation energy
K: Boltzmann constant
T: Temperature in kelvin
The process of excitation and decay to ground state is involved in the two techniques of atomic spectroscopy.
We measure the energy absorbed or emitted and use it for quantification process
Atomic Spectroscopy Methods
- Atomic Emission Spectroscopy (Flame Photometry)
- Atomic Absorption Spectrometry (AAS)
Atomic Emission Spectroscopy (AES) (Flame Photometry)
Principle: Flame photometry is based upon those particles that are electronically excited in the medium.
Flame: is the source of excitation energy. (Low energy source).
Uses: Flame photometry is used mainly for the determination of alkali metals and easily excited elements (Na, K, Li, Ca, etc.) particularly in biological fluids and tissues
Functions of Flame
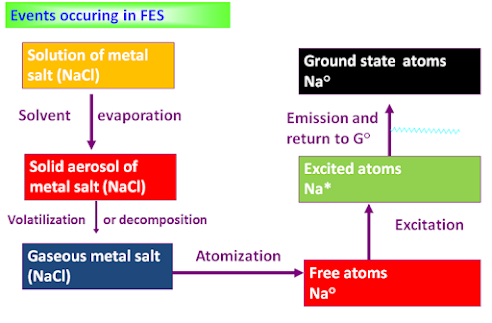
1. To convert the constituents of liquid sample into the vapor state.
2. To decompose the constituents into atoms or simple molecules:
M+ + e– (from flame) -> M + hn
3. To electronically excite a fraction of the resulting atomic or molecular species
M -> M*
The flame is composed of: a fuel gas and oxidant gas
| Oxidant – Fuel | Max. Temp. (oC) |
| Air- propane | 1725 |
| Air- acetylene | 2400 |
| Oxygen- acetylene | 3100 |
| Nitrous oxide-acetylene | 3000 |
| Air-hydrogen | 2000 |
| Oxygen-hydrogen | 2700 |
| Air + argon -hydrogen | 1577 |
Factors affecting the intensity of flame emission:
1- The concentration of the analyte in solution
2- The rate at which excited atoms are formed in the flame.
3- The rate at which the sample is introduced into the flame.
4- Temperature of the flame.
5- Composition of the flame.
6- The ratio of fuel to oxidant in the flame.
7- Solvent used to dissolve the sample.
The flame temperature is the most important factor. Increase in flame temperature causes an increase in emission intensity. This is controlled by composition of the flame.
High temperature flames should not be used for elements that ionized easily e.g. Na, K, Li or Ce. However, high temperature flames are generally favored for transition elements and alkaline earth metals.
Effect of the solvent used to dissolve the sample; if the solvent is water the process is slow and if it is organic solvent the process is fast and emission intensity is increased.
It is therefore very important that calibration curves be prepared using the same solvent.
The stochiometric ratio of fuel to oxidant in the flame must be used, in which both fuel to oxidant are totally consumed.
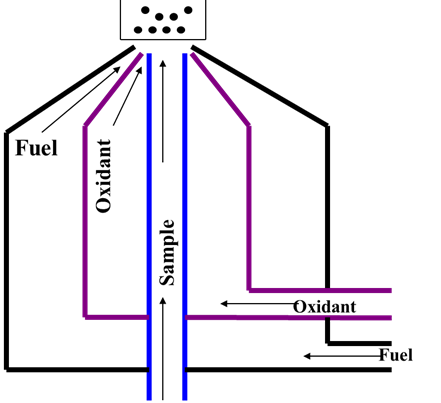
The nebulizer-burner system
To convert the test sample into gaseous atoms
Nebulizer produces an aerosol of the test solution
Burner system – the mixing of fuel and oxidant for flame
Types of burner system
1. Pre-mix or laminar flow burner
2. Total consumption burner
1. Pre-mix or laminar flow burner
Advantages
1. Homogenous flame
2. Suitable for AAS and AES as the pathway could be
increased
Disadvantages
Suffers from explosion hazards
2. Total consumption burner
3 concentric tubes, the sample, fuel and oxidant only mix at the tip of burner
Used mainly for FES (short path)
Advantages
1. Simple to manufacture
2. Allows a total representative sample to reach the flame
3. Free from explosion hazards
Disadvantages
1. Aspiration rate varies with different solvents
2. Suitable only for AES
Non Flame Atomizers
For example: Heated Granite Furnace
Sample evaporation→ time and temp. Controlled drying and ashing
Advantages
1. Small samples are analysed
2. 1000-fold more sensitive than flame
3. Oven is adaptable for determination of solid samples
Disadvantages
1. Low accuracy
2. Low precision
3. More ionic interferences due to very high temp.
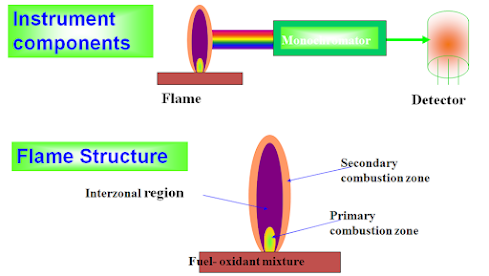
Monochromators Detectors Analytical technique
As in UV photomultipliers
Choice of the wavelength: of max. Sensitivity and min. spectral interferences
Sample preparation:
It is very important to obtain the sample in a form of solution, where the spectral and chemical interferences are absent
Demineralized dist. Water and very pure reagents are to be used because of the high sensitivity of the technique
Because of the instability of the very dil. Solution, it is advisable to dilute the soln just before use.
Several elements can be determined in blood, urine, cerebrospinal fluid and other biological fluids by direct aspiration of the sample after dilution with water.
Chemical interferences: can often be overcome by simple dilution with a suitable reagent solution e.g. serum is diluted by EDTA solution for the determination of calcium to prevent interference from phosphate.
Standard curves
Deviations from linearity may occur
Qualitative analysis
Flame photometry is useful mostly for the detection of elements in group I and II of the periodic table. The presence of certain elements can be detected by the use of a filter or monochromator.
Quantitative analysis
To perform quantitative analysis, the sample is introduced into the flame and the intensity of radiation is measured. The concentration of the emitting substance is then calculated from a calibration curve or using standard addition method.
Application of flame photometry in pharmaceutical analysis
1. Metals are major constituents of several pharmaceuticals such as dialysis solutions, lithium carbonate tablets, antacids and multivitamin-mineral tablets.
2. The elements Na, K, Li, Mg, Ca, Al and Zn are among the most common elements subjected to pharmaceutical analysis using flame emission technique.
3. Sodium and potassium levels in biological fluids are difficult to analyze by titrimetric or colorimetric techniques. Their analysis is very important for control of infusion and dialysis solutions which must be carefully monitored to maintain proper electrolyte balance.
Advantages:
1. Flame emission is the simplest and least expensive technique.
2. The analysis may be carried out without prior separation as other components such as dextrose, do not interfere.
Summary
• Flame photometry is an example of atomic and emission spectroscopy
• Nebulizing burner systems are the unique components found in flame photometers.
• Premix or laminar flow type and complete combustion type nebulizer–burner systems are the two types of burner systems used in flame photometers
• Flame photometry is used determine the elements of I and II groups of the periodic table
For PDF Notes Click on the Download Button