PILOT PLANT SCALE UP TECHNIQUES

PILOT PLANT SCALE UP TECHNIQUES
Pilot Plant Scale Up
In every emerging pharmaceutical industry or an already existing one, there is always a need to have an intermediate batch scale representing procedures and simulating that used for commercial manufacturing
This is achieved by determining the ability of formula to withstand batch-scale and process modification
R & D à PILOT SCALE à SCALE UP à LARGE SCALE MANUFACTURE
What is Pilot plant??
“Defined as a part of the pharmaceutical industry where a lab scale formula is transformed into a viable product by the development of liable practical procedure for manufacture.”
Why conduct Pilot Plant Studies?
A pilot plant investigates a product and process on an intermediate scale before large amounts of money are committed to full-scale production
It is usually not possible to predict the effects of a many-fold increase in scale
It is not possible to design a large complex food processing plant from laboratory data alone with any degree of success
A pilot plant can be used for
Evaluating the results of laboratory studies and making product and process corrections and improvements
Producing small quantities of product for sensory, chemical, microbiological evaluations, limited market testing or furnishing samples to potential customers, shelf-live and storage stability studies
Determining possible salable by-products or waste stream requiring treatment before discharge
Providing data that can be used in making a decision on whether or not to proceed to a full scale production process; and in the case of a positive decision, designing and constructing a full-size plant or modifying an existing plant
Considerations in pilot plant development
Kind and size – depends on goals; evaluating product and process; producing samples of product for evaluation; market testing or furnishing to potential customer
Location: near R&D facility? At an existing plant? Close liaison between R&D and pilot plant staff is essential
Labor requirements and costs: engineering staff, skilled operations and maintenance staff
Pilot plant costs may exceed those of usual plant production costs. The pilot plant may be used for training personnel for a full- scale plant
Scale-up: – The art for designing of prototype using the data obtained from the pilot plant model
Pilot plant scale-up techniques involve reproducible manufacture of an experimental formulation on high-speed production equipment, in a cost effective manner
It is a part of the pharmaceutical industry where the same processes used during Research and Development (R&D) of dosage forms are applied to different output volumes; usually greater than that obtained during R&D
PILOT SCALE
â
INTERMEDIATE BATCH SCALE
â
Representative and simulates Manufacturing scale
SCALE UP
â
NEXT TO THE PILOT SCALE
â
Process of increasing the batch size/ procedure for applying the same process to different output volumes
Objective of scale up
“Find mistakes on small scale and make profit on large scale.”
To produce physically and chemically stable therapeutic dosage forms
Review of the processing equipment
Guidelines for productions and process control
Evaluation and validation
To identify the critical features of the process.
To provide master manufacturing formula.
Pilot plant scale up must include:
Close examination of the formula to determine its ability to withstand batch scale process modification
Compatibility of the equipment with the formulation
Cost factor
Availability of raw materials meeting the specifications required to produce the product
Market requirement
Physical space required and the layout of the related functions
Pilot Plant Design
A pilot plant design should support three key strategic
Objectives:
1. Formulation and process development
2. Clinical supply manufacture
3. Technology evaluation, scale up and transfer
Attributes playing a key role in achieving the above objectives are:
cGMP Compliance
A flexible highly trained staff
Equipment to support multiple dosage form development
Equipment at multiple scales based on similar operating principles to those in production
General considerations
1. Personnel Requirements:
Personnel should have –-
Scientists with experience in pilot plant operations as well as in actual production area (to understand the intent of the formulator as well as understand the perspective of the production personnel
The group should have some personnel with engineering knowledge as well as scale up also involves engineering principles
2. Space Requirements
Administrative and information processing
Physical testing area
Standard equipment floor space
Storage area
2a. Administrative and Information processing
Adequate office and desk space should be provided for both scientist and technicians
The space should be adjacent to the working area
2b. Physical testing area
This area should provide permanent bench top space for routinely used physical- testing equipment
2c. Standard equipment floor space
Discreet pilot plant space, where the equipment needed for manufacturing all types of dosage form is located.
Intermediate – sized and full scale production equipment is essential in evaluating the effects of scale-up of research formulations and processes.
Equipment’s used should be made portable where ever possible so that after use it can be stored in the small store room
Space for cleaning of the equipment should be also provided
2d. Storage area
Two separate areas for approved and unapproved active ingredient as well as excipients
Separate areas for the storage of the in-process materials, finished bulk products from the pilot-plant & materials from the experimental scale-up batches made in the production, packing material
3. Review of the formula
A thorough review of the each aspect of formulation
The purpose of each ingredient and its contribution to the final product manufactured on the small-scale laboratory equipment
Effect of scale-up using equipment that may subject the product to stresses of different types and degrees can more readily be predicted, or recognized.
4. Raw Materials
One purpose/responsibility of the pilot-plant is the approval & validation of the active ingredient & excipients raw materials.
Why?
Raw materials used in the small scale production cannot necessarily be the representative for the large scale production
5. Relevant Processing Equipment
The most economical and the simplest & efficient equipment which are capable of producing product within the proposed specifications are used
The size of the equipment should be such that the experimental trials run should be relevant to the production sized batches
If the equipment is too small the process developed will not scale up, whereas if equipment is too big then the wastage of the expensive active ingredients
6. Production Rates
It can be determined by the immediate future market requirements
Equipment and the process should be chosen on the basis of production of a batch at a frequency that takes into consideration:
1. Product loss in the equipment during manufacture
2. The time required to clean the equipment between batches
3. The number of batches that will need to be tested for release
7. Process Evaluation
It is the basis of process validation
Documentation of process is to be done
Process is validated only if there are no changes in the formula, quality of the ingredients, or the equipment configuration
Revalidation needs to be done to ensure that changes have not taken place
8. Preparation of Master Manufacturing Procedures
Manufacturing directions
Sampling directions
In process QC
Final product QC
Helps in better understanding by the technician and compliance
9. Product stability and uniformity
• The primary objective of the pilot plant is the physical as well as chemical stability of the products
• Hence each pilot batch representing the final formulation and manufacturing procedure should be studied for stability
• Stability studies should be carried out in finished packages as well
10. GMP considerations
GMP items that should be a part of scale up are –
✓ Equipment qualification
✓ Process validation
✓ Regularly schedule preventative maintenance
✓ Regularly process review & revalidation
✓ Relevant written standard operating procedures
✓ The use of competent technically qualified personnel
✓ Adequate provision for training of personnel
✓ A well-defined technology transfer system
✓ Validated cleaning procedures.
✓ An orderly arrangement of equipment so as to ease material flow & prevent cross contamination
11. Transfer of Analytic methods to Quality Assurance
• During scale up the analytical test methods developed in research should be transferred to the QA dept.
• QA staff to review process to make sure proper analytic equipment’s are available and personnel are trained
• They should review the assay procedures and the that obtained during validation studies to verify that the there are no changes in the analytical procedure
Challenges in Scale-Up
Scaling up production comes with challenges:
Homogeneity: Achieving uniformity in larger batches can be challenging, particularly with chemical reactions.
Equipment Adaptation: Adapting laboratory or pilot-scale equipment to larger-scale operations may require significant modifications.
Quality Assurance: Maintaining stringent quality control throughout the scale-up process is essential.
FAQ
What is pilot plant scale-up in industrial processes?
Pilot plant scale-up refers to the process of transitioning from a small-scale operation, such as a laboratory or pilot plant, to a larger commercial-scale production facility while maintaining product quality and consistency.
Why is pilot plant scale-up important in various industries?
Pilot plant scale-up is essential because it allows industries to efficiently produce larger quantities of products, reduces costs, maintains quality, meets regulatory requirements, and ensures timely market availability.
What are the key techniques for successful pilot plant scale-up?
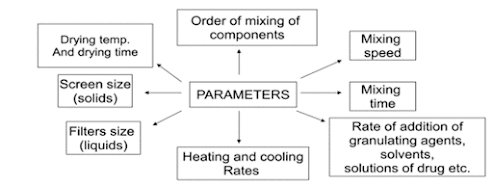
Key techniques include understanding the process, assessing scalability, process optimization, equipment selection, and continuous monitoring of critical parameters.
How can one assess the scalability of a process during scale-up?
Assessing scalability involves evaluating whether the process can be successfully replicated on a larger scale without compromising product quality, efficiency, or safety.
What challenges are commonly encountered during pilot plant scale-up?
Challenges may include achieving homogeneity in larger batches, adapting equipment to larger-scale operations, and maintaining stringent quality control throughout the scale-up process.
Why is continuous monitoring of critical parameters important during scale-up?
Continuous monitoring helps ensure that the process remains within specified parameters, which is crucial for maintaining product quality and consistency at a larger scale.
Can you provide examples of industries that frequently utilize pilot plant scale-up techniques?
Industries such as pharmaceuticals, chemicals, food processing, and manufacturing often employ pilot plant scale-up techniques to transition from small-scale to commercial production.
Are there specific case studies that illustrate successful pilot plant scale-up?
Yes, case studies provide real-world examples of successful scale-up projects, showcasing the strategies and techniques used to achieve a smooth transition to larger-scale production.
For Detailed PDF Notes Click on Download Button