Pro-drugs
• Initially
used by Albert
• Is
a pharmacologically inactive compound that is converted into an active drug by
a metabolic biotransformation
• Can
be enzymatic/non-enzymatic
• Non-enzymatic
such as hydrolysis- compounds may cause stability problems
• Conversion
can occur before ADME or at specific site in the body
• Soft
drug- pharmacologically active and uses metabolism for promotion of excretion
Why prodrug
Lead modification approach used to correct a flaw in drug
candidate
• Aqueous
solubility
• Absorption
and distribution
• Site
specificity
• Instability
• Prolonged
release
• Toxicity
• Poor
patient acceptability
• Formulation
problems
Types of prodrugs
• A)
Carrier linked prodrugs and B) Bioprecursors
• A)
Carrier linked prodrugs- active drug linked to a carrier group
• Carrier
group- should be labile, non-toxic, biologically inactive
• Further
divided to bipartate, tripartate and mutual prodrugs
• Bipartate-
prodrug with carrier
• Tripartate-
carrier + linker + prodrug
• Mutual
prodrug- synergistic drugs connected to each other
• B)
Bioprecursors- compound metabolized by molecular modification into new compound
which can be drug
• No
resemblance to desired functional group
• Drastic
structural change is required to unmask desired group
• Oxidation
is common metabolic biotransformation
Carrier linked prodrugs
• An
ideal drug carrier must
• (1)
protect the drug until it is at the site of action;
• (2)
localize the drug at the site of action;
• (3)
allow for release of the drug chemically or enzymatically;
• (4)
minimize host toxicity;
• (5)
biodegradable, biochemically inert, and non-immunogenic;
• (6)
be easily prepared inexpensively; and
• (7)
be chemically and biochemically stable in its dosage form
• Most
common (biologically labile) functional groups utilized in prodrug design are
shown above.
Prodrug Active Form of Drug
• Esters
are the most commonly employed prodrugs.
• Numerous
catalytic esterases are present in vivo to hydrolyze simple esters.
• However,
different species have differing amounts and types of esterases with different
substrate specificities and different rates of hydrolysis.
• This
can make it difficult for pharmaceutical companies to generate accurate
preclinical models in which to evaluate their candidate prodrug.
• One
example is the monoethyl ester of enalaprilat, which is called enalapril.
• Enalaprilate
(upper left) was first discovered as an inhibitor of angiotensin converting
enzyme (ACE) and used to treat hypertension.
• Due
to its high polarity, note two COOH’s, it was not orally bioavailable, and thus
needed to be administered by injection.
• The
monomethyl ester, enalapril (upper right) is orally bioavailable.
• Another
example is the anti-viral agent Oseltamavir (Tamiflu®) shown above
• Notice
that the oral bioavailability is improved by employing the ethyl ester of the
carboxylic acid
Famciclovir
• Such
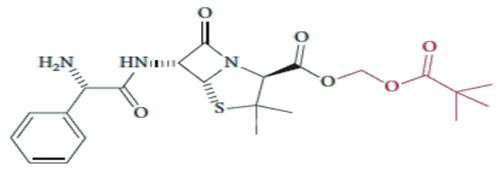
a strategy is employed for pivampicillin, as shown above.
• Such
a strategy can also be used to (temporarily) convert phosphate groups into more
lipophilic ester moieties, as shown above.
• Increased
water solubility
Bioprecursors
• Activation
of leflunomide to active drug