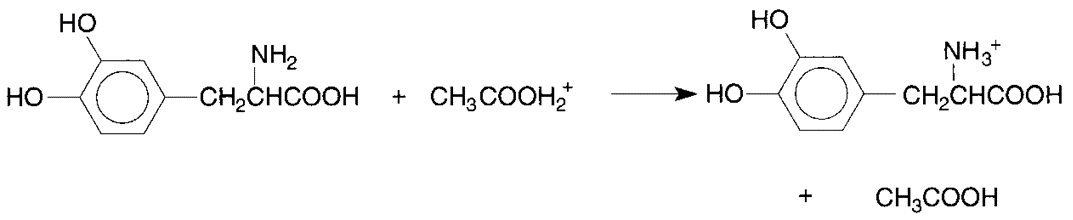Non Aqueous titration

Learning Objectives
At the end of this lecture, the student will be able to
• Explain the theory of non-aqueous titration
• Discuss about the solvents used in non-aqueous titrations
• Define and classify the different types non aqueous titration
• Preparation and standardisation of 0.1M perchloric acid
• Outline the applications of non-aqueous titration
Introduction
Some compounds posed two vital problems of quality control, both in pure and dosage forms by virtue of their inherent characteristics, namely:
• Poor solubility, and
• Weak reactivity in aqueous medium
Such compounds were estimated with great difficulty
• Example 1: Amine salts—It is first changed to the water-soluble free base, extracted with an appropriate-organic solvent and treated with an excess volume of standard acid; subsequently, the solvent was evaporated, and the remaining acid determined with a standard base.
• Example 2: Sodium salts—It is first acidified to release the water- insoluble organic acid, extracted with a suitable organic solvent, the solvent was removed and the residue was subsequently dried and weighed.
• Example 3: Nitrogen containing compounds—They are estimated by micro Kjeldahl’s Method.
Nevertheless, such specific quantitative methods gave rise to certain serious anomalies and drawbacks
In order to overcome these shortcomings the non-aqueous titrations were introduced
• Non-aqueous titrations have the following advantages, namely:
Elimination of poor solubility of substances
Enhancement of weak reactivity of substances
Selective titration by using suitable solvent and titrant of acidic/basic components of physiologically active moiety of a salt
• Maintenance of speed, precision, accuracy and simplicity at par with classical methods of analysis
• Weak bases which have Kb values less than 10–6 can be titrated satisfactorily by non-aqueous titrations
• The reason being that in aqueous medium and at higher Kb values (> 10–6) the solvent water competes progressively with the basic species in solution for the proton of the solvent
Theory of Non Aqueous Titration
The theory of non-aqueous titration is based on Lowry-Bronsted theory
According to this theory:
• An acid is a proton donor and
• A base is a proton acceptor
Therefore,
• When an acid HA undergoes dissociation it gives rise to a proton and the conjugate base A of the acid:
HA ó H+ + A–
Acid Proton Base
• In other words, the liberated base A shall unite with a proton to give the corresponding conjugate acid HA of the base A because every base has its conjugate acid and vice versa
• Hence, from the above definitions it may be implied that:
(a) An acid: could be either an electrically neutral molecule: e.g., HNO3; or a negatively charged anion e.g., HSO4–; or a positively charged cation e.g., C6H5NH +, H3O+;
(b) A base: could be either an electrically neutral molecule e.g., C6H5NH2; or an anion e.g., Cl–, NO3–
Note: Substances which are potentially acidic can function as acids only in the presence of a base to which they can donate proton
Solvents Used in Non-Aqueous Titration
Different types of solvents used in non-aqueous titration are classified as:
• Aprotic solvents
• Protophilic solvents
• Protogenic solvents
• Amphiprotic solvents
Aprotic solvents:
– Neutral
– Chemically inert
– Low dielectric constant
– Do not react with acids or bases. So do not favor ionization
Examples: Toluene and chloroform
Protophilic solvents:
• Basic in character and reacts with acids to form solvated protons
HB + Sol ó Sol.H+ + B-
Acid basic Solvent Solvated proton conjugate base
2 Differentiation – levelling effect (Kd ~ 10-12 − can be measured)
a) Differentiation effect:
In water: HClO4 ≈ HCl ≈ HNO3
In CH3COOH: HClO4 > HCl > HNO3
In HF: medium > weak > base
acid
Conclusion:
Strong acids (in water) can separetely be measured in acidic solvents
Strong bases can separately be measured in basic solvents
b) Levelling effect:
In Water: HCl > CH3COOH > benzoic acid
In pyridine: HCl ≈ CH3COOH ≈ benzoic acid
Conclusions:
Weak acid (in water) can be measure in basic solvents
Weak bases can separately be measure in acidic solvents
Protogenic solvents
• Are acidic substances
• They exert a leveling effect on bases
Examples: Sulfuric acid
Amphiprotic solvents
• Have both
– Protophilic and
– Protogenic properties
Examples: Water, Glacial acetic acid, Alcohols
Explanation: How glacial acetic acid act as amphiprotic solvents?
HCLO4 + Pyridine in glacial acetic acid
acid: HClO4 + CH3COOH ó ClO4– + CH3COOH2
base: Py + CH3COOH ó PyH+ + CH3COO−
ClO4 − + CH3COOH2+ + PyH + CH3COO– ó PyH+…ClO4– + 2CH3COOH
Advantages of Using Non-Aqueous Solvents
• Determination of organic acids and bases which have a limited solubility in water.
Disadvantages of Using Non-Aqueous Solvents
• Expensive
• Volatile
• Toxic
• Removal of water is necessary, can take water (humidity) from the air
Choice of Solvents in Non-Aqueous Titration
• Pure, nontoxic, miscible with titrant solute
• High dielectric constant
• Depending upon the solubility and nature of sample under investigation
– For weak acid— basic solvents like DMF, pyrdine are used
– For weak base—- acidic solvent like glacial acetic acid is used
• No side reactions between the sample and titrant
• The solvent chosen should not affect the sharpness of endpoint during titration
Examples of Non-Aqueous Solvents
Glacial Ethanoic Acid:
• The most frequently used non-aqueous solvent
• Before it is used it is advisable to check the water content. This may be between 0.1% and 1.0%
Dimethylformamide:
• It is a protophillic solvent, which is frequently employed for titrations between, for instance, benzoic acid and amides
• Although end points may sometimes be difficult to obtain
Acetonitrile: (methyl cyanide, cyanomethane)
• It is frequently used with other solvents such as chloroform and phenol and especially with ethanoic acid
• It enables very sharp end points to be obtained in the titration of metal ethanoates when titrated with perchloric acid
Dioxane:
• It is another popular solvent, which is often used in place of glacial ethanoic acid when mixtures of substances are to be quantified
• Unlike ethanoic acid, dioxane is not a levelling solvent and separate end points are normally possible, corresponding to the individual components in the mixtures
Alcohol:
• Salts of organic acids, especially of soaps are best determined in mixtures of glycols and alcohols or mixtures of glycols and hydrocarbons.
• The most common combinations are ethyleneglycol (dihydroxyethane) with propan-2-ol or butan-1-ol
• The combinations provide admirable solvents for both the polar and non-polar ends of the molecules
Indicators Used in Non-Aqueous Titration
Indicator | Color change | Color change | Color change |
| Basic | Neutral | Acidic |
Crystal violet (0.5 per cent in glacial acetic acid) | violet | blue-green | yellowish- green |
α-Naphtholbenzein (0.2 per cent in glacial acetic acid) | blue or blue- green | orange | dark-green |
Oracet Blue B (0.5 per cent in glacial acetic acid) | blue | purple | pink |
Quinaldine Red (0.1 per cent in methanol) | magenta | almost colorless |
Non Aqueous Titration: Classification
Classification in general:
• Acidimetry: Weakly basic substances: alkali salts of organic acids, amines, amine salt, heterocyclic nitrogen
Titrant: used is acetous perchloric acid
Solvents: glacial acetic acid, acetic anhydride
Indicators: Crystal violet, methyl rosaniline, quinaldine red
• Alkalimetry: Weakly acidic substances: sodium benzoate, sulfanilamides
Titrant: Sodium methoxide, lithium methoxide, sodium amino methoxide
Solvents: DMF, pyridine, ethylenediamine,n-butyl amine, morpholine
Indicators: Quinaline red, thymol blue Azoviolet, O-nitroaniline
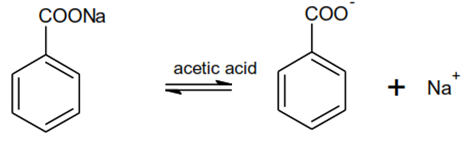
Titration of Alkali Metal Salts of Organic Acids
Application: Preparation of 0.1M Perchloric acid
Slowly add 8.5 ml of 72% perchloric acid
â
900 ml of glacial acetic acid with continuous and efficient mixing
â
30 ml of acetic anhydride
â
Adjust the final volume to 1000 ml with glacial acetic acid
â
Allow the solution to stand for 24 h before use
Role of acetic anhydride is to remove the water molecule
• Alkali and alkaline earth salts of organic acids function as bases in acetic acid solution
RCOOM ó RCOO- + M+
CH3COOH2 + ó RCOOH + CH3COOH
Hence potassium hydrogen phthalate is used for standardising acetous perchloric acid
Standardization of 0.1 M perchloric acid
Dilute up to the mark with glacial acetic acid
Warm until salt has dissolved
Add glacial acetic acid about 25ml
Accurately weigh (0.5g) of potassium hydrogen phthalate into a 100 ml volumetric flask
Cool and titrate against 0.1M acetous perchloric acid using 2 drops of 0.5%w/V of acetous crystal violet until blue changes to blue green
Reactions Involved in Standardization of Perchloric acid
HClO4 + CH3COOH óCH3COOH2 + + ClO4−
CH3COOH2 + + CH3COO– ó 2 CH3COOH
KHC8H4O4 + HClO4 ó C8H6O4 + KClO4
Estimation of Sodium benzoate
HClO4 ó H+ + ClO4–
CH3COOH + H+ ó CH3COOH2+
•Titration Nonaqueous titration
•Method Acidimetry
•Indicator Crystal violet
•End point Violet to emerald green
Titration of Amines and Amine Salts of Organic Acids
• A wide range of primary, secondary and tertiary amines can be assayed by acetous per chloric acid
Examples: Adernaline, Chlordiazepoxide, Metronidazole — etc
Example: Adernaline
Titration of Primary Amines
Example: Methlyldopa
R.NH2 + HClO4 → [R.NH3] + + ClO4–
• Specific reaction between methyldopa and perchloric acid is expressed by the following equation
Titration of Halogen Acid Salts of Bases
• The halide ions chloride, bromide and iodide are too weakly basic to react quantitatively with acetous perchloric acid
• Addition of mercuric acetate to a halide salt replaces the halide ion by an equivalent quantity of acetate ion, which is a strong base in acetic acid
Example: Assay of ephedrine hydrochloride
Chemical reaction involved in above method
2R.NH2.HCl ó 2RNH3 + + 2 Cl–
(CH3COO) 2 Hg ( Undissociated) + 2 Cl – →HgCl2 (Undissociated) + 2 CH3COO–
2CH3COOH2 + 2CH3COO- ó 4CH3COOH
Examples: Ephedrine hydrochloride, Chloropromazine hydrochloride —etc
Example –Ephedrine hydrochloride
Alkalimetry:
• Weakly acidic substances are determined by non-aqueous titration using potassium, sodium or lithium methoxide in toluene-methanol
• Or alternatively with tetrabutylammonium hydroxide in methanol
• Above titrant is used in the assay of cyclic imides (Ethosuximide), thiazides Hydrochlorthiazide), sulphonamides (Sulphafuruzole) and phenols (Dichlorophen)
Preparation of 0.1 N Sodium Methoxide
• Material Required: Absolute methanol, dry toluene, Potassium metal.
• Procedure:
Add into a dry flask, a mixture of methanol (40 ml) and dry toluene (50 ml) and cover it loosely
Carefully add freshly cut pieces of sodium metal (2.3 gm) to the above mixture gradually with constant shaking. After complete dissolution of potassium metal
Add enough absolute methanol to yield a clear solution
Toluene 50 ml is added with constant shaking until the mixture turns hazy in appearance
The process is repeated by the alternate addition of methanol and benzene until 1 litre of solution is obtained, taking care to add a minimum volume of methanol to give a visible clear solution
Standardization of 0.1N Sodium Methoxide
• Material Required: Dimethylformamide (DMF): 10 ml; thymol blue (0.3% in MeOH); 0.1 N lithium methoxide in toluene methanol; benzoic acid: 0.6 g.
• Procedure:
Transfer 10 ml of DMF in a conical flask and add to it 3 to 4 drops of thymol blue
And first neutralize the acidic impurities present in DMF by titrating with 0.1 N lithium methoxide in toluene-methanol
Quickly introduce 0.06g of benzoic acid and titrate immediately
with methoxide in toluene-methanol
• Caution: Care must be taken to avoid contamination of neutralized liquid with atmospheric carbon dioxide
Chemical Equations: The various equations involved in the above operations are summarized as stated below:
(i) Na + CH3OH → CH3ONa + H↑
Precaution: It is an exothermic reaction and hence, special care must be taken while adding the metal into the dry solvent in small lots at intervals with adequate cooling so as to keep the reaction well under control.
(ii) H2O + CH3ONa → CH3OH + NaOH
H2CO3 + 2CH3ONa → 2CH3OH + Na2CO3
Precaution: The clear solution of sodium methoxide must be kept away from moisture and atmospheric CO2 as far as possible so as to avoid the above two chemical reactions that might ultimately result into the formation of turbidity.
Chemical equations: For Standardization
Step 1: It shows the solution of benzoic acid (primary standard) in DMF
C6H5COOH + H—CON(CH3)2 ↔ HCON+H(CH3)2+C6 H5 COO-
DMF
Step 2: It depicts ionization of sodium methoxide
CH3ONa ↔ CH3O- + Na+
Step 3: It illustrates the interaction between the solvated proton and the methylated ion
HCONH+(CH3)2 + CH3O – → HCON(CH3)2 + CH3OH
Summarised whole equation is:
C6H5COOH + CH3ONa → C6H5COONa + CH3OH
In summing up, the net reaction between the water in the solvent (DMF) and the titrant is equivalent to the volume of sodium methoxide consumed by DMF or may be considered as a blank determination.
Advantages of Non Aqueous Titrations
1) Organic acids and bases that are insoluble in water are soluble in non- aqueous solvent
2) Organic acid, which is of comparable strength to water, can be titrated easily in non-aqueous solvent. Bases also follow the same rules
3) A non-aqueous solvent may help two are more acids in mixture. The individual acid can give separate end point in different solvent
4) By the proper choice of the solvents or indicator, the biological ingredients of a substance whether acidic or basic can be selectively titrated
5) Non aqueous titrations are simple and accurate, examples of non-aqueous titration are: Ephedrine preparations, codeine phosphate in APC, tetracycline, teramycin, Antihistamines and various piperazine preparations
Applications of Non Aqueous Titration
Sodium acetate
CH3COONa + HClO4 → CH3COOH + NaClO4
Chemical equation for standardisation:
HClO4 + CH3COOH ó CH3COOH2 + + ClO4−
CH3COOH2 + + CH3COO– ó 2 CH3COOH
KHC8H4O4 + HClO4 ó C8H6O4 + KClO4
Indicator used in non-aqueous titration
Name of Substance | Indicator Employed |
Amantadine hydrochloride | Crystal violet |
Chlorpromazine hydrochloride | Methyl orange |
Clonidine hydrochloride | α -Naphthol benzein |
Cyproheptadiene.HCl | Crystal violet |
Ephedrinehydrochloride | Crystal violet |
Acetazolamide | Potentiometric determination |
Bendrofluazide | Azo violet |
Allopurinol | Thymol blue |
Mercaptopurine | Thymol blue |
Amylobarbitone | Quinaldine Red |
Nalidixic acid | Thymolphthalein |
Summary:
• Non aqueous titrations: applied for estimation of weak acidic or basic substances and water insoluble substances
• Theory of non-aqueous titration is based on Bronsted Lowry concept
• Different types of solvent used are: Aprotic, protophilic, protogenic and amphiprotic solvents
• Indicators: Crystal violet, α-Naphtholbenzein, Oracet Blue B, Quinaldine Red
• Types of non-aqueous titration applied for different compounds:
1. Alkalimetry
2. Acidimetry
3. Titration of alkali metal salts of organic acids
4. Titration of amines and amine salts of organic acids
5. Titration of halogen acid salts of bases
• Perchloric acid can be standardised by using potassium hydrogen phthalate
• Applications: Sodium benzoate, ephedrine hydrochloride, Phenobarbitone
Also, Visit: B. Pharmacy Notes | B. Pharma Notes | Study material Bachelor of Pharmacy