Precipitation titration

Learning Objectives
At the end of this lecture, the student will be able to
• Define precipitation titration
• Classify precipitation titration
• Explain Mohr’s method of precipitation
• Explain Volhard’s method
• Explain Modified Volhard’s method
• Explain Modified Fajans method
• Outline the application of precipitation titration
Precipitation Titration
• Type of volumetric analysis that depends on the formation of a precipitate
• Precipitating agent used in precipitation titration is Silver Nitrate (Argentometric titration).
• Application:
-Used for determination of halides (Cl-, Br- & I-),
-Used for determination of thiocyanide and cyanide, –Some compounds that form insoluble products (ppt) with Silver Nitrate
Requirements for a Precipitation Titrations
• The precipitate must be practically insoluble
• The precipitate should be rapid
• The reaction should go rapidly to completion and should be quantitatively
• Only one reaction should take place
• The titration results should not be hampered by adsorption (Co precipitate) effects
• The compounds must be ionic in nature
• There should be no change or any harm in detecting the end point
Reason for Less Popularity of This Titration
• Lack of suitable indicators to detect the end point in precipitation titration
• The composition of the precipitate is not always known
• The cost silver nitrate is too high
Methods of Determining the Equivalence Point in Precipitating Titration
• Mohr’s method – Formation of colored precipitate
• Volhard’s method- Formation of Colored complex ions (Colored solution)
• Modified Volhard’s method
• Fajan or Adsorption indicator method- Depends on the formation of Colored adsorption compound
Mohr’s Method (1856)
• Sample: Cl- , Br- (Not for I- or SCN-).
• Type of titration: Direct titration.
• Standard Solution: Standard silver nitrate solution.
• pH: Neutral or slightly alkaline. (pH ≈ 6.5 -9).
• Indicator: 1 ml of 5% Potassium Chromate (K2CrO4) soln.
• Color at end point: Brick red ppt due to formation of Silver chromate (Ag2CrO4)
• Titration reaction:
Cl– + Ag + → AgCl ↓ Ksp = 1.8 x 10–10
sample titrant white ppt
• Endpoint reaction
2 Ag++ CrO4– – → Ag2CrO4 ↓ Ksp = 1.2 x 10–12
titrant indicator brick red ppt
Limitations of Mohr’s Method
1. Effect of pH: Mohr’s method should be done in neutral or slightly alkaline medium (pH ≈ 6.5-9) because:
-At pH > 9: Ag+ will be precipitated as AgOH↓ (brown to black ppt): leading to Consumption of the titrant & Masking of the end point color.
Ag+ + OH– → AgOH ↓ (brown to black ppt)
-While at pH < 6.5: The chromate ion (CrO4 –) changes into acid chromate (HCrO4 -) then to
dichromate (Cr2O7 –). Both HCrO4 – & Cr2O7 — form soluble salts with Ag+ & so no colored ppt will be formed at the end point.
2 CrO4 — + 2 H+ → 2 HCrO4 – → Cr2O7 — + H2O
chromate acid chromate dichromate
2. Ag2CrO4 is more soluble than AgCl so that no Ag2CrO4 will be precipitated until all Cl- ions have been precipitated as AgCl provided that CrO4– concentration should be adjusted to make Ag2CrO4 formed only at the end point and so to prevent error in the end point, 1 ml of 5% K2CrO4 solution is suitable based on solubility product.
• High concentration of K2CrO4 ⇒ gives too soon (early) E.P. because Ag2CrO4 will be rapidly precipitated before E.P.
• Low concentration of K2CrO4 ⇒ gives too late E.P. because CrO4—will be insufficient and so a large amount of Ag+ (titrant) will be needed to precipitate Ag2CrO4 and so the E.P. comes too late.
3. I– & SCN– cannot be determined by Mohr’s method because the formed silver chloride & silver thiocyanate strongly adsorb CrO4— on their surfaces and so the ppt formed at the end point will be an adsorption compound which is less colored and so less sharp endpoint will be obtained
Modification of Mohr’s method
1. Silver chromate – soluble in acid, therefore the solution to be titrated –neutral. If acidic, first neutralized –pure powdered calcium carbonate and then indicator is added
2. Solution – alkaline – form ppt of silver hydroxide, first neutralized by adding dilute nitric acid. Then the excess acid is neutralized – pure powdered calcium carbonate
Sodium chloride estimation
Equipment:
Burette and stand
10 and 20 mL pipettes
100 mL volumetric flask
250 mL conical flasks
10 mL and 100 mL measuring cylinders
Solutions Needed:
Silver nitrate solution: (0.1 mol L−1) If possible, dry 5 g of AgNO3 for 2 hours at 100°C and allow to cool. Accurately weigh about 4.25 g of solid AgNO3 and dissolve it in 250 mL of distilled water in a conical flask. Store the solution in a brown bottle.
Potassium chromate indicator solution: (approximately 0.25 molL-1) Dissolve 1 g of K2CrO4 in 20 mL distilled
Titration:
1. Dilute seawater by pipetting a 20 mL sample into a 100 mL volumetric flask and making it up to the mark with distilled water.
2. Pipette a 10 mL aliquot of diluted seawater into a conical flask and add about 50 mL distilled water and 1 mL of chromate indicator.
3. Titrate the sample with 0.1 mol L−1 silver nitrate solution. Although the silver chloride that forms is a white precipitate, the chromate indicator initially gives the cloudy solution a faint lemon-yellow colour
4. Repeat the titration with further aliquots of diluted seawater until concordant results (titres agreeing within 0.1 mL) are obtained.
Result Calculation
1. Determine the average volume of silver nitrate used from your concordant titres.
2. Calculate the moles of silver nitrate reacting.
3. Use the following reaction equation to determine the moles of chloride ions reacting.
Ag+(aq) + Cl– (aq) → AgCl(s)
4. Calculate the concentration of chloride ions in the diluted seawater.
5. Calculate the concentration of chloride ions in the original undiluted seawater.
6. Calculate the concentration of sodium chloride in the seawater in molL−1, gL−1 and g/100 mL(%).
Volhard’s Method (1874)
• Sample: Cl- , Br- I- or SCN-.
• Type of titration: Back titration.
• Standard Solution: Standard ammonium thiocyanate solution.
• pH: Acidic
• Indicator: ferric alum.
• Color at end point: Brick red ppt due to formation of ferrous thiocyanate complex
Steps involved in Volhard’s method
Step 1: Adding excess Ag+ into sample
Ag+ + Cl– → AgCl↓ + left Ag+
Step 2: Removing AgCl ↓ by filtration/washing
Step 3: Adding Fe3+ into filtrate (i.e., the left Ag+)
Step 4: Titrating the left Ag+ by SCN–:
Ag+ + SCN– → AgSCN ↓
Step 5: End point determination by red colored Fe(SCN)2+ complex. (when all Ag+ has been consumed, SCN– reacts with Fe3+)
SCN– + Fe3+ → Fe(SCN)2+(aq)
Disadvantages of Volhard’s method
• During the filtration process, the sample may be lost
• Filtration process has be performed in order to prevent the interaction of precipitated silver chloride with thiocyanate ions.
• In case of iodide estimation, iron (III) indicator should not be added until excess of silver is present, since the dissolved iodide reacts with iron (III) ions
2 Fe3+ + 2I- ó 2Fe2+ + I2
Modified Volhard’s method
In order to prevent the reaction between precipitated silver chloride and thiocyanate and to avoid the filtration process:
– A coating reagent is added in the reaction such as nitrobenzene, dibutyl phthalate, potassium nitrate Such method is known as modified Volhard’s method
Fajan’s method
• Fajan’s method is the most recent and most accurate silverhalide method
• It is based on the adsorption of adsorption indicators on the surface of the positively charged silver chloride particles formed in the precipitation titration when Ag+ ion is in excess
• Sample: Generally, it can be used for Cl-, Br-, I- & SCN-.
[Fluorescein ind. is used for Cl-, Br-, I- & SCN- but Eosin ind. is used for Br-, I-& SCN-(Not for Cl-)]
• Type of titration: direct titration.
• Standard Solution: Standard silver nitrate solution.
• Indicator: Adsorption indicators like eosin, fluoroscein…
• Color at end point: Depending upon the use of indicator
Adsorption Indicators
• Based on the properties of colloids adsorption indicators act
• An adsorption indicator is an organic compound (dye) that tends to be adsorbed onto the surface of the solid in a precipitation titration.
• Ideally, the adsorption occurs near the equivalence point
• And results not only in a color change but also in a transfer of color from the solution to the solid (or the reverse).
Examples:
Acid dyes: Fluorescein, eosin as sodium salts
Basic dyes: Rhodamine 6G and Phenosafranine as halogen salts
Theory of Adsorption Indicators
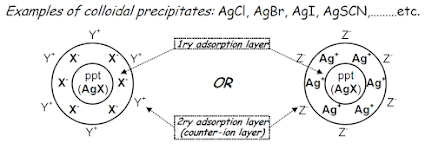
• The action of adsorption indicators depends on the electrical phenomena associated with the formation of colloidal precipitates
• Colloidal ppt tends to form an electrical double layer around its particles when it is in contact with a solution. This electrical double layer consists of:
a) Primary adsorption layer: in this layer, the ppt tends to adsorb one of its own ions (the ion present in excess).
b) Secondary adsorption layer: a layer of opposite charge (counter-ion layer) containing oppositely charged ions from the surrounding solution
Determination of sodium chloride by using adsorption indicator method
• It depends on the titration of NaCl sample with standard AgNO3 using fluorescein indicator.
• At the endpoint, the formed AgCl↓ ppt becomes pink.
• Titration reaction:
NaCl + AgNO3 fluorescein ind AgCl ↓ + NaNO3
Explanation:
Preparation and Standardization of Silver Nitrate Solution
Preparation:
• Silver Nitrate, 0.1 M: Dissolve 17.0 g in sufficient water to produce 1000 ml.
Standardization:
• Weigh accurately about 0.1 g of sodium chloride, previously dried at 110° for 2 hours and dissolve in 5 ml of water.
• Add 5 ml of acetic acid, 50 ml of methanol and 0.15 ml of eosin solution. Stir, preferably with magnetic stirrer, and titrate with the silver nitrate solution.
I.P factor: 1 ml of 0.1 M silver nitrate is equivalent to 0.005844 g of NaCl.
Preparation and Standardization of Ammonium Thiocyanate
Preparation:
• Ammonium Thiocyanate, 0.1 M: Dissolve 7.612 g of ammonium thiocyanate in sufficient water to produce 1000 ml.
Standardization:
• Pipette 30.0 ml of 0.1 M silver nitrate into a glass-stoppered flask
• Dilute with 50 ml of water, add 2 ml of nitric acid and 2 ml of ferric ammonium sulphate solution and
• Titrate with the ammonium thiocyanate solution to the first appearance of a red-brown colour.
I.P factor: 1 ml of 0.1 M silver nitrate is equivalent 0.007612 g of NH4SCN.
Assay of Sodium Chloride Based on Modified Volhard’s Method
Step 1: Adding excess Silver nitrate solution into sample in acidic media using nitric acid, ferric alum and dibutyl phthalate:
AgNO3 + NaCl → AgCl↓ + NaNO3 + AgNO3
Step 2: Titrate the left out/unreacted silver nitrate with ammonium thiocyanate :
AgNO3 + NH4 SCN → AgSCN ↓+ NH4NO3
Step 3: End point determination by red colored Fe(SCN)2+ complex. (when all Ag+ has been consumed,
SCN– reacts with Fe3+)
SCN– + Fe3+ → Fe(SCN)2+(aq)
Assay procedure as per I.P 2007
Assay:
• Weigh accurately about 0.1 g and dissolve in 50 ml of water in a glass-stoppered flask.
• Add 50.0 ml of 0.1 M silver nitrate, 5 ml of 2 M nitric acid and 2 ml of dibutyl phthalate, shake well and
• Titrate with 0.1 M ammonium thiocyanate using 2 ml of ferric ammonium sulphate solution as indicator, until the colour becomes reddish yellow.
I.P.Factor: 1 ml of 0.1 M silver nitrate is equivalent to 0.005844 g of NaCl.
Summary:
• Type of volumetric analysis that depends on the formation of a precipitate
• Mainly used for estimating halides with the help of silver nitrate or ammonium thiocyanate
• Different methods: Mohr’s, Volhards, Fajans
• Different methods: Volhards, Fajans
• Volhards method are carried out in acidic media with back titration process
• Fajans method is based on adsorption indicator method
• Application mainly used to estimate sodium chloride and potassium chloride in electrolytes
Also, Visit: B. Pharmacy Notes | B. Pharma Notes | Study material Bachelor of Pharmacy