Potentiometery

Indicator Electrodes
• To measure potential of a solution, an appropriate electrode is needed
• Indicator electrode measures potential when connected to a suitable reference electrode
• Electrode which is used to measure potential or pH of a solution is called as indicator electrode
Types of indicator electrode used are:
• Hydrogen electrode
• Quinhydrone electrode
• Antimony electrode
• Glass electrode
Hydrogen Electrode
• Construction is similar to Normal Hydrogen Electrode (NHE)
• Electrode is similar to NHE
• Electrode is responsive to hydrogen ion concentration
• Can be used for pH measurement
• Platinum of electrode do not take part in electrochemical reaction
• Only acts as site for transfer of electrons
• Potential of hydrogen electron is
• E H+, H2 = E0(H+, H2= a) – 0.0591/n (H+)
=0.0591 pH, as E0(H+, H2= a) = 0
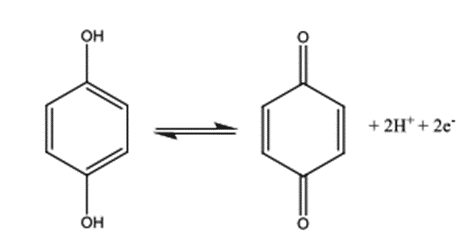
Quinhydrone Electrode
• Electrode is used in measuring pH of a given solution
• Consists of a bright platinum wire dipping into a solution saturated with quinhydrone
• In solution, quinhydrone dissociates into equimolecular quantities of quinone and hydroquinone
• pH of a solution can be determined by a redox system
• Because reduction to hydroquinone or oxidation to benzoquinone involves H+ ions
Pros
• Attains equilibrium rapidly
• Can be used where hydrogen electrode is unsuitable
• Gives accurate results
Cons
• Cannot be used in more alkaline solution, whose pH is above 8
• Gets readily oxidized by air in alkaline medium
Antimony Electrode
• Used in pH measurements
• Consists of rod coated or covered with antimony trioxide
• Electrode is prepared by placing a stick or a rod of antimony metal covered with thin crust of its oxide
• It is dipped into a solution whose pH is to be measured
• Electrical connection is made with reference electrode like saturated calomel electrode
• Reaction involved is
• Sb + H2O === SbO+ + 2H+ + 3e
• Sb2O3 + 6H+ + 6e === 2Sb(s) + 3H2O
Pros
• Can be used for measuring pH from 2 to 8
• Can be used in viscous or turbid solvents
• It is sturdy- very useful where continuous pH recording are made
• Do not get readily poisoned
Cons
• Cannot be used in measuring pH below 3, as oxide gets dissolved
• Cannot be used in the presence of strong oxidizing and complexing agents
• Cannot be used in the presence of metals such as Cu, Ag, Au which are more noble than antimony (i.e., below in electromotive series)
• It suffers from salt error
Glass Electrode
• Very useful electrode for determination of hydrogen ion (or pH) of a solution
• It involves no electron exchange but allows transfer of H+ ions through its membrane
• Consists of very thin bulb made from glass membrane having high electrical conductivity
• Bulb contains hydrochloric acid solution of definite concentration and or Ag/AgCl wire to make electrical contract with it
• Thin membrane of bulb is prepared from special type of glass, sensitive and permeable to H+ ions
• Glass membrane is made from soft soda-lime glass containing lithium silicate with lanthanum and barium ions added to it
• Sensitivity of membrane of protons and other cations depend upon the composition of glass membrane
• Corning glass contains 22% Na2O, 6% CaO and 72% SiO2
• Membrane of glass is specific to H+ ions upto pH 9.0 above which it is somewhat responsive to Na+ and other charged cations
• Potential of glass electrode is given by
• E = k + 0.0592 (pH1-pH2) at 25 0C
• Where k = constant for the electrode
• pH1 = pH of the solution inside the bulb
• pH2 = pH of the test solution (outside)
• E = k + 0.0592 pH1 – 0.0592 pH2
• E = K – 0.0592 pH2
• Since pH1 = constant, k + 0.0592 pH1 is also another constant
Pros
• Gives a rapid response
• It is chemically resistant to oxidizing and reducing agents, dissolved gases, colloids and salts, etc
• Can be used over the entire pH range when lithia-silica glasses are used
Cons
• Extremely fragile- contains a very thin bulb
• Minute abrasions on the surface of tip, damages electrode
Note
• Never be allowed to remain dry
• All glass electrodes must be conditioned by soaking in water or saturated potassium chloride or dilute acid buffer solution
Potentiometric Titrations
• Performed for the solutions which show changes in potential or pH by addition of a reagent or titrant
• Here the absolute value of potential with respect to standard half-cell is not required to be known
• Changes occurring during the course of addition of titrant are sufficient
• Equivalence point of reaction is shown by sudden change in potential on a plot of emf readings against the volume of titrant being added
• Knowledge of actual potential of reference electrode need not be known accurately
• Any electrode which will furnish a constant potential isuseful as a reference electrode
• Provided its potential remains constant throughout the titrations
Potentiometery Apparatus and Requirements
• Can be done manually or under automation
• If it is manually, a beaker with stirrer and a pipette are sufficient
• In case of automated models,
• A sample cell which can hold a pair of electrode
• Inlet for titrant
• Stirrer for mixing the solution are essential
• Pair of electrodes depends on the type of titration i.e., acid-base or redox
• In most titrations, saturated calomel electrode is used as reference electrode
• Indicator electrode varies with respect to titrant
• Suitable indicator electrode is used
• For example, glass electrode for acid-base titrations
• Platinum for redox titrations
Pros
• Colored solutions, dilute solutions or turbid suspensions can be titrated
• Actual potential of reference electrode need not be known
• Titration can be automated
• Inexpensive method with more accuracy
Method of Detecting End Point
• When indicator method is not suitable, we use potentiometric method for determining end point
• A normal titration curve i.e. a plot of
• emf vs volume of titrant or
• pH vs volume of titrant
• First derivative curve i.e. a plot of ΔE/ Δv or ΔpH/ Δv vs volume of titrant
• Second derivative curve i.e. a plot of Δ2E/Δv2 or Δ2pH/Δv2 vs volume of titrant
• In a potentiometric titration, at the end point
• Rate of change of potential is maximum
Applications
• Acid-base titrations
• Redox titrations
• Diazotisation titrations
• Precipitation titrations
• Complexometric titrations
• Dead stop end point technique
Applications of Potentiometery
• Analysts make more potentiometric measurements than any other chemical instrumental measurement
• Manufacturers measure the pH of many consumer products
• Clinical laboratories determine blood gases as important indicators of disease states
• Industrial and municipal effluents are monitored continuously to determine pH and concentration of pollutants
• Oceanographers determine carbon dioxide and other related variables in sea water
• Also used in fundamental studies to determine equilibrium constants such as Ka, Kb
• Thousands of applications for potentiometric measurements are available
Electrochemistry
• Analyst always ask questions such as
• What is it?
• How much of it is present?
• How fast does it change?
• Electrochemistry is ideal analytical tool for answering these questions
• Can be termed as electroanalysis
• Determines the potential of electrochemical cells- usually at zero current
• Potential of electrode responds to change in concentration of species under study
• With respect to standard reference electrode
• Most common potentiometric methods used by analyst employ pH meters
• Relatively cheap to perform
• But can be slow and tedious unless automated
• Electrochemical cell comprises of two electrodes
• Indicator electrode- voltage of which solely depends on concentration of one specific component in the solution
• Reference electrode- voltage of which must be absolutely independent of the nature and composition of solution
Nernst Equation
• The potential of a metal electrode at 25 0C immersed into a solution of its own ions is given by
E = E0 + 0.0592/n log c
• Where E0 – standard potential of the metal
• n- valency of ions
• c- concentration of ions
Components of a Potentiometric Cell
1. Reference electrode
2. Salt bridge
3. Analyte
4. Indicator electrode
• Electrodes are relatively free from interferences
• Provides rapid, convenient and nondestructive means for quantitative determination of numerous important anions and cations
• Placing together of these two electrodes on a solution gives rise to an electrochemical cell
• From this voltage generated across the electrodes may be determined by connecting to a potentiometer
• Instrument should be sensitive to measure ± 0.2mV
• Under these experimental parameters, the e.m.f. of the cell may be expressed as
• Ecell = E+ – E- + Ej
• Ej is the e.m.f. at the liquid junction
Reference Electrodes
Ideal reference electrode-
• Has a potential that is accurately known and constant
• Completely insensitive to composition of the analyte solution
• Electrode should be rugged
• Easy to assemble
• Should maintain a constant potential while passing minimal currents
• For example, calomel reference electrodes
• Consists of mercury in contact with a solution that is saturated with mercury chloride (calomel) and also contains a known concentration of potassium chloride
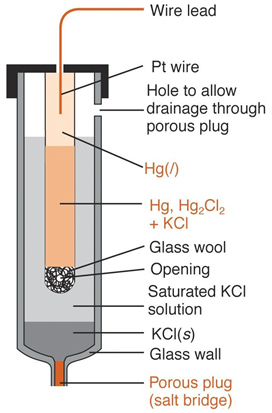
Calomel Reference Electrode
• Calomel half-cells can be represented as
• Hg|Hg2Cl2 (saturated), KCl(xM)||
• X represents the molar concentration of potassium chloride in the solution
• Electrode potential for this half-cell is determined by the reaction
• Hg2Cl2(s) + 2e- ó 2Hg + (l) + 2Cl-(aq)
• Calomel electrode is commercially available as
• H-shape body of the electrode is made of glass
• Right arm of electrode contains a platinum electrical contact
• Small quantity of Hg|Hg2Cl2 paste in saturated potassium chloride and few crystals of potassium chloride
• The tube is filled with saturated KCl to act as a salt bridge through a piece of porous Vycor (thirsty glass) sealed in the end of left arm
Saturated Calomel Electrode
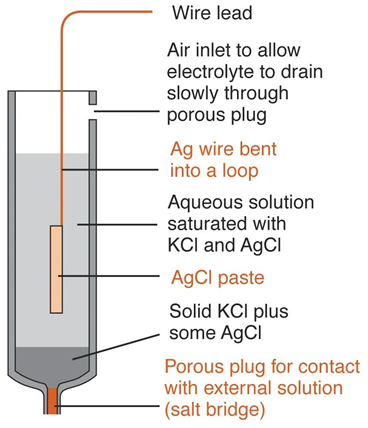
Silver/Silver Chloride Reference Electrode
• Most widely marketed reference electrode
• Consists of a silver electrode immersed in a solution of potassium chloride that has been saturated with silver chloride
• Represented as Ag|AgCl(saturated), KCl(saturated)||
• Electrode potential is determined by the half-reaction
• AgCl(s) + e- ó Ag(s) + Cl-
• Electrode is prepared with either a saturated or 3.5M potassium chloride solution
• It is a glass tubing with narrow opening at the bottom connected to a Vycor plug for making contact with analyte solution
• Tube contains a silver wire coated with a layer of silver chloride that is immersed in a potassium chloride solution saturated with silver chloride
• Advantage- can be used at temperature greater than 600C, while calomel electrodes cannot
• Mercury ions react with fewer sample components
• Such reactions can lead to plugging of the junction between electrolyte and the analyte solution
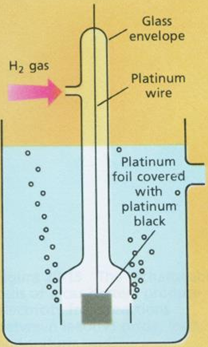
Standard Hydrogen Electrode
• Hydrogen gas electrode
• Pt(H2(1 atm), H+ (1M)
Construction:
• Consists of a glass tube having holes at its bottom
• Inside this tube is another glass tube having a platinum or copper wire with a platinum foil
• Surface of platinum foil is coated electrically with platinum black
• Pure hydrogen gas at 1 atmospheric pressure is passed through opening of glass tube
• And it escapes through small holes at the bottom of the electrode
• Electrode is dipped in a solution of standard acid like hydrochloric acid of unit activity (1.8 M of HCl at 25 0C)
• Some hydrogen gas is absorbed by platinum black of electrode and it permits the exchange from gaseous to ionic of hydrogen
• Also reverse the process to occur without any obstacle
• Under fixed conditions of pressure of hydrogen gas passed and hydrogen ions in solution with
contact of electrode, hydrogen electrode possess a definite potential
• By convention, this potential is taken as zero at all temperature
• When normal hydrogen electrode is connected to any electrode through salt bridge, potential of that electrode can be measured
• For example, zinc electrode (zinc metal rod in contact with a solution of zinc ion) is connected by potassium chloride salt bridge, can be represented as
• Pt, H2 |H+ (a=1)|| Zn+2 || Zn
• Where a is unknown
• Cell reaction is
• H2 + Zn+2 à 2H+ (a=1) + Zn
• Similar way, we can measure the potential of any electrode
• Half-cell reactions of electrode are written as
• Mn+ + ne- === M or
• Zn+2 + 2e- === Zn, for zinc E0 = 0.76 volts
• Electrode potential E is equal to standard potential E0, when activity of Mn+ is equal to unity (1M solution)
• Potential of a hydrogen electrode depends upon the pH value of a solution which it is contact
• pH value of a given solution can be calculated by combining with normal hydrogen electrode (NHE) or with any standard reference electrode like saturated calomel electrode
• Combined cell reaction can be represented as,
• Pt, H2 (1 atm), H+unknown ||KCl(sat)Hg2Cl2(s), Hg
• E of above reaction is Ecell = Ecalomel(sat) – Ehydrogen
Advantages:
• Fundamental electrode and is used as standard in pH measurements
• Can be used over wide pH range
• Exhibits no salt error
• Establishes equilibrium rapidly and gives accurate results
Disadvantages
• Cannot be used in solutions containing strong oxidizing or reducing agents
• Cannot be used in solutions containing metal ions that below hydrogen in potential series
• Interaction with hydrogen will occur and the metal will be deposited on electrode surface
• Gets readily poisoned by number of substances like proteins, tannins, mercury salts, etc
• Cumbersome to prepare and use in routine analysis
• Because of its cumbersomeness, it is replaced by other standard electrodes
SUMMARY
• Types of indicator electrodes used
• Hydrogen electrode, Quinhydrone electrode, Antimony electrode, Glass electrode
• Equivalence point of reaction is shown by sudden change in potential on a plot of emf readings against the volume of titrant being added
• In most titrations, saturated calomel electrode is used as reference electrode
• When indicator method is not suitable, we use potentiometric method for determining end point
• Analysts make more potentiometric measurements than any other chemical instrumental measurement
• Determines the potential of electrochemical cells- usually at zero current
• Potential of electrode responds to change in concentration of species under study
• Most common potentiometric methods used by analyst employ pH meters
• Relatively cheap to perform
• Indicator electrode- voltage of which solely depends on concentration of one specific component in the solution
• Reference electrode- voltage of which must be absolutely independent of the nature and composition of solution
• Nernst equation- E = E0 + 0.0592/n log c
Also, Visit: B. Pharmacy Notes | B. Pharma Notes | Study material Bachelor of Pharmacy