Conductometry

Objectives
By the end of this session, students will be able to:
• Define conductometry
• Define and explain the principle involved in conductometric titrations
• Discuss the pros and cons Conductometry
• Explain precautions to be taken for conductometric titrations
• Brief the applications of conductometric titrations
Conductometry
• Measurement of conductivity of a solution
• Due to mobility of cations and anions towards respective electrodes
• Conductivity (C) is inversely proportional to resistance (R) of a solution
C = 1/R
• Unit of conductivity is mhos or ohms-1
• Conductivity of a solution depends upon-
Number of ions (concentration)
Charge of ions
Size of ions
Temperature
• Resistance of a solution is given by
R = E/I
Where E = potential difference
I = current which flows through
Unit of resistance (R) is ohms
Potential difference (E) is volts
Current (I) is amperes
• Resistance of a solution depends upon length (l) and cross resistance (a) of the conductor through which conductivity takes place
R = ρl/a
• ρ is specific resistance
• Specific resistance (ρ) is the resistance offered by a substance of 1cm length and 1 sq.cm surface area, Unit of measurement is ohm cm
• Specific conductivity (kv) is the conductivity offered by a substance of 1cm length and 1 sq.cm surface area, Unit of measurement is mhos cm-1
• Equivalent conductivity (λv) is the conductivity of a solution containing equivalent weight of the solute between electrodes 1 cm and 1 sq.cm, Unit of measurement is mhos cm-1
• Molar conductivity (μv) is the conductivity of a solution containing molecular weight of the solute between electrodes 1 cm apart and 1 sq.cm surface area
• Molar conductivity = specific conductivity x volume of solution containing one molecular weight of the electrolyte
Measurement of Conductivity
• Conductivity may be measured by applying an alternating electrical current (I) to two electrodes immersed in a solution and measuring the resulting voltage (V)
• Cations migrate to the negative electrode, the anions to the positive electrode and the solution acts as an electrical conductor
• Conductivity is typically measured in aqueous solutions of electrolytes/ ions
• For the actual determination of conductivity, we need
• Wheatstone bridge circuit and Conductivity cell
• Conductivity cells are of different types
• Made up of platinum and coated with platinum black
• If the electrodes are old, platinisation can be done be done by using 3% solution of chloroplatinic acid and 0.02-0.03% of lead acetate to get uniform coating
• Different electrodes used depends upon the conductivity of the solution is high or low
• Commonly used are platinum electrodes
• Wheatstone bridge circuit consists of
• Standard resistance in one of its arms
• Other arm contains a conductivity cell (platinum electrode) dipped into the solution whose conductivity is to be determined
• Galvanometer shows the deflection of standard resistance with that of resistance of unknown solution
• R2/R1 = Resistance of BC/ Resistance of BA
• R2 is resistance of unknown solution
• R1 is standard resistance
• R2 = BC/BA x R1
• Conductivity of unknown solution = BA/BC x R1
• Observed conductivity is not always the specific conductivity
• Dimensions of the platinum electrode of various manufacturers are not same
• Distance between the electrodes and surface area of electrodes varies
• Value of cell constant to be calculated
• Cell constant (x) = l/a
• Where l = distance between electrodes
• a = area of electrode
• Relation between specific conductivity and observed conductivity can be derived as R = ρl/a
• R/ρ = l/a
• x = R/ρ = 1/observed conductivity / 1/specific conductivity
• x = R/ρ = specific conductivity / observed conductivity
• Specific conductivity = x * observed conductivity
• Specific conductivity = cell constant x observed conductivity
• Determination of cell constant
• Cell constant of a conductivity cell is determined by measuring the conductivity of a known strength of potassium chloride at specific temperature
• Conductivity of 0.02 KCl at 25 0C, cell constant is
• 2765/ observed conductivity of 0.02 KCl at 25 0C in µmhos
• Conductivity of 0.01 KCl at 25 0C, cell constant is
• 1221/ observed conductivity of 0.02 KCl at 25 0C in µmhos
Conductometric Titrations
• End point determination by conductivity measurements
• Conductivity solution depends on
• Change in number of ions
• Mobility of ions
• Graph of conductivity vs volume of titrant added
Pros
• Determination of specific conductivity is not required
• Not necessary to use conductivity water
• No indicator is required
• Titrations can be done with colored or dilute or turbid solutions
• Incompletion at end point doesn’t affect results as measurements before and after end point are sufficient
• End point is determined graphically, errors are minimized and can get accurate end point
• Cell constant need not be determined provided the same electrode is used throughout the experiment
• Temperature need not be known provided it is maintained constant throughout the titration
Apparatus required
• Titration vessel (beaker)
• Stirrer for mixing
• Automatic or manual burette to deliver titrant
• Conductivity meter with a conductivity cell (platinum electrode)
Procedure
• Conductivity is measured in millimhos or micromhos
• Titrant is added in small increments like 0.5 ml – 1.0 ml
• Solution is mixed properly and conductivity readings are taken
• Readings were taken before and after end point
• Graph is plotted- conductivity vs volume of titrant added
• Point of intersection is found
• Corresponds to end point or volume of titrant required to neutralize the reactants or sample present in titration vessel
Precautions to be taken
• Initial volume of titrating substance and final volume after titration are not same
• Conductivity measurements made during titration are subject to error
• Correction factor is included to know actual conductivity
• Actual conductivity = observed conductivity X (𝑖𝑛𝑖𝑡𝑖𝑎𝑙 𝑣𝑜𝑙𝑢𝑚𝑒 + 𝑣𝑜𝑙.𝑜𝑓 𝑡𝑖𝑡𝑟𝑎𝑛𝑡 𝑎𝑑𝑑𝑒𝑑 /𝑖𝑛𝑖𝑡𝑎𝑙 𝑣𝑜𝑙𝑢𝑚𝑒)
• Temperature should be maintained constant
• Heat of neutralization may affect the temperature and it effects the conductivity of solution
Acid base Titrations
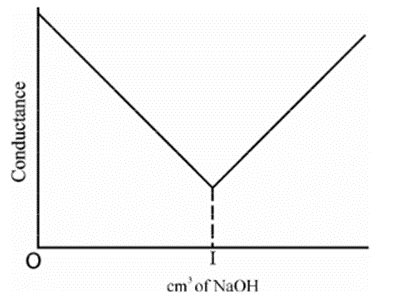
Strong acid vs Strong base
• HCl + NaOH à NaCl + H2O
• HCl in beaker as titrate- high conductivity
• Strong acid- dissociation is complete
• NaOH is added as titrant – conductivity gradually decreases after every addition
• After the end point, when all the H+ has reacted- addition of NaOH increases the concentration of OH- ions – conductivity starts increasing
• First part of curve shows steep fall in conductivity because of decrease in H+ ions
• Second part of curve shows gradual increase because of increase in OH- ions Strong Acid vs Weak Base
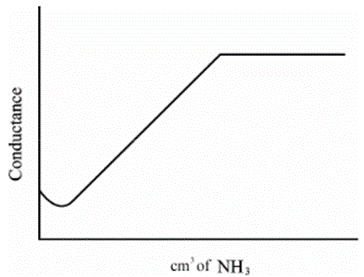
Strong Acid vs Weak Base
• HCl + NH4OH à NH4Cl + H2O
• HCl in beaker as titrate- high conductivity
• Strong acid- dissociation is complete
• NH4OH is added as titrant – conductivity gradually decreases after every addition
• After the end point, when all the H+ has reacted- addition of NH4OH doesn’t cause increase in the concentration of OH- ions
• Poor dissociation- conductivity remains constant
• First part of curve shows steep fall in conductivity because of decrease in H+ ions
• Second part of curve shows plateau
Weak Acid vs Strong Base
• CH3COOH + NaOH à CH3COONa + H2O
• CH3COOH in beaker as titrate- initial conductivity is low
• Weak acid- doesn’t dissociate into H+ ions
• NaOH is added as titrant – slight increase in conductivity till end point
• After the end point, addition of NaOH causes increase in the concentration of OH- ions
• Conductivity starts to increase steeply
• First part of curve shows gradual increase
• Second part of curve shows steep increase because of increase in OH- ions
Weak Acid vs Weak Base
• CH3COOH + NH4OH à CH3COONH4 + H2O
• CH3COOH in beaker as titrate- initial conductivity is low
• Weak acid- doesn’t dissociate into H+ ions
• NH4OH is added as titrant – ammonium acetate salt has better conductivity gradually increases after every addition
• After the end point, when all the CH3COOH has reacted addition of NH4OH causes no increase in the conductivity
• Plateau is obtained
• First part of curve shows gradual increase in conductivity because of ammonium acetate salt
• Second part of curve shows plateau because or poor dissociation of NH4OH
Comparison between Potentiometric and Conductometric titration
|
|
Potentiometric titration |
Conductometric titration |
|
Parameter measured |
Potential in mv |
Conductivity in mhos |
|
Parameter not necessary |
Potential of reference electrode |
Cell constant |
|
At end point
|
Rate of change of potential is maximum |
Sharp change in conductivity occurs |
|
End point determination |
Normal curve, first derived curve, second derivative curve |
End point shown by intersection of two lines |
|
Strength of titrant |
Same as that of titrate |
5 or 10 times stronger than titrate |
|
Dependency |
Temperature dependent |
Temperature dependent |
Applications
• Solubility of sparingly soluble salts- silver chloride, barium sulfate, lead sulfate
• Ionic product of water
• Basicity of organic acids- number of carboxylic groups present in the molecule- tartaric acid, oxalic acid
• Purity of water- specific conductivity of pure water is 5 x 10-8 ohm-1 cm-1
• Quantitative analysis
• Salinity of sea water
• Equilibrium in ionic reactions- progress of ionic reactions can be determined
Summary
• Measurement of conductivity of a solution- Due to mobility of cations and anions towards respective electrodes
• Resistance of a solution depends upon length (l) and cross resistance (a) of the conductor through which conductivity takes place
• Conductivity may be measured by applying an alternating electrical current (I) to two electrodes immersed in a solution and measuring the resulting voltage (V)
• Cations migrate to the negative electrode, the anions to the positive electrode and the solution acts as an electrical conductor
• Observed conductivity is not always the specific conductivity
Conductivity solution depends on
• Change in number of ions
• Mobility of ions
• Graph of conductivity vs volume of titrant added
• Conductivity is measured in millimhos or micromhos
Frequently asked questions (FAQs)
1. What is conductometry, and what are its fundamental principles?
Conductometry is a technique used to measure the electrical conductivity of a solution. It is based on the principle that the ability of a solution to conduct electricity is directly related to the concentration of ions in the solution.
2. How is the conductivity of a solution measured in conductometry?
Conductivity is measured using a conductometer, which consists of a conductivity cell with two electrodes. The cell is immersed in the solution, and the electrical conductivity is determined by measuring the electrical resistance between the electrodes.
3. What are the advantages and disadvantages of conductometric titrations in analytical chemistry?
Pros of conductometric titrations include their speed, precision, and suitability for a wide range of analytes. Cons may include sensitivity to electrode contamination and the need for carefully prepared standard solutions.
4. What precautions should be taken when conducting conductometric titrations?
Precautions include ensuring clean and dry electrodes, avoiding contamination of the solution, calibrating the instrument regularly, and maintaining a stable temperature.
5. Can you outline the general procedure for a conductometric titration?
The procedure involves preparing the sample and titrant, immersing the conductivity cell in the sample, adding the titrant, and monitoring the change in conductivity until the endpoint is reached.
6. How does potentiometry differ from conductometry in analytical chemistry?
Potentiometry measures the potential difference (voltage) between two electrodes in a chemical cell, while conductometry measures the electrical conductivity of a solution. Potentiometry is often used for measurements involving non-conductive species.
7. What are the typical applications of conductometry in various fields?
Conductometry is used in a range of applications, including environmental monitoring, water quality analysis, chemical process control, and pharmaceutical quality control, among others.
8. Can you explain the role of conductometry in environmental monitoring and assessment?
Conductometry is valuable in environmental analysis, particularly for measuring the salinity of water bodies, detecting contamination by ions, and evaluating the impact of pollutants in natural waters.
9. How does temperature affect conductometric measurements, and what measures can be taken to address this effect?
Temperature can influence the conductivity of solutions. Compensation for temperature effects can be achieved by using a temperature-controlled cell or applying correction factors based on the sample’s temperature.
10. Are there any limitations to the accuracy of conductometric measurements?
The accuracy of conductometric measurements can be affected by factors like impurities in the electrodes, variations in temperature, and the presence of complex ions. Regular maintenance and calibration can help improve accuracy.
Also, Visit: B. Pharmacy Notes | B. Pharma Notes | Study material Bachelor of Pharmacy