Biosynthesis of Purine

Objective
• At the end of this lecture, student will be able to
• Explain biosynthesis of purine nucleotides
• Explain salvage pathway
• Describe degradation of purine nucleotides
• Discuss consequences of defective purine metabolism
Biosynthesis of purine nucleotides
• Two major routes for purine nucleotide biosynthesis
• Many compounds contribute to purine ring of nucleotides
• N1 of purine is derived from Aspartate
• C2 & C8 arise from N10-Formyl THF
• N3 &N9 from glutamine
• C4, C5 & C7 from glycine
• C6 directly comes from CO2
• Major site of purine synthesis is in the liver
• Purines are actually synthesised as ribonucleotides
• Synthesis of the purine nucleotides begins with PRPP and leads to the first fully formed nucleotide, IMP
• The purine base without the attached ribose moiety is hypoxanthine
• The purine base is built upon the ribose by several amidotransferase and transformylation reactions
• Synthesis of IMP requires five moles of ATP, two moles of glutamine, one mole of glycine, one mole of CO2, one mole of aspartate and two moles of formate
- Liver is the major organ for purine nucleotide synthesis
- Ribose 5P obtained by carbohydrate metabolism is the precursor for synthesis of purine nucleotides. It react with ATP to form Phosphoribosyl pyrophosphate (PRPP) in presence of PRPP synthetase
- Glutamine transfers its amide to PRPP to replace pyrophosphate & produce β-5-phosphoribosyl amine
- Phosphoribosyl amine react with glycine in presence of ATP to form glycin amide ribosy-5-phosphate
- N10 formyl THF donate the formyl group & produce formyl glycin amide ribosyl -5- phosphate
- Glutamine transfer the second amide group to form formyl glycin amide ribosyl -5- phosphate
- The imidazole ring is closed in an ATP dependent reaction to yield 5-amino-imidazole ribosyl -5- phosphate
- Incorporation of Co2 occurs to yield amino imidazole carboxylate ribosyl -5- phosphate
- Aspartate condenses to form amino imidazole-4- succinyl carboxamide ribosyl -5- phosphate
- Adenosuccinatelyase cleaves of fumarate and only the amino group of aspartate is retained to yield amino imidazole -4- carboxamide ribosyl -5- phosphate
- N10-Formyl THF donate a one carbon moiety to produce 5 formyl amino imidazole -4- carboxamide ribosyl-5- posphate . With this reaction all the carbon & nitrogen atoms of purines ring are obtained
- Formyl amino imidazole -4-carboxamide ribosyl-5- phosphate catalysed by cyclohydrolase and leads to close ring with elimination of H2O molecule to form Inosine monophosphate
- IMP is the immediate precursor for formation of GMP & AMP
- Aspartate condenses with IMP in presence of GTP to produce Adenyl succinate which cleavage to form AMP
- IMP undergoes NAD dependent dehydrogenation to form AMP and glutamine then combines with XMP and forms GMP.
• IMP (parent nucleotide) does not accumulate in cells but is rapidly converted to other purine nucleoside monophosphates AMP (adenosine monophosphate) & GMP (guanosine monophosphate)
• IMP represents a branch point for purine biosynthesis, because it can be converted into either AMP or GMP through two distinct reaction pathways
• The pathway leading to AMP requires energy in the form of GTP; that leading to GMP requires energy in the form of ATP
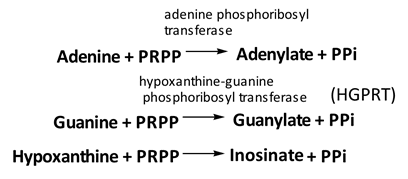
Salvage pathway
Phosphoribosyl transferases involved in salvage pathway convert free bases to nucleotides
Purines can be directly converted to the corresponding nucleotides
• Regulation of purine biosynthesis is based on the availability of intracellular concentration of PRPP, PRPP synthase and ribose -5- Phosphate
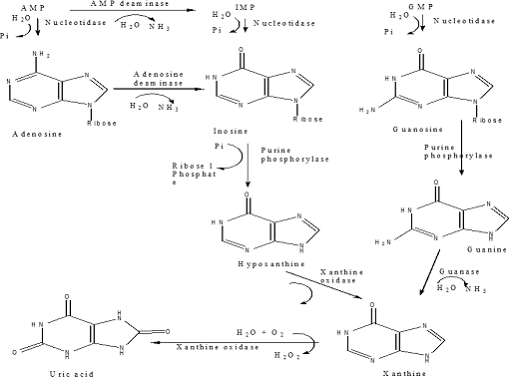
Catabolism of Purine nucleotides
Disorders of defective purine metabolism
1. Hyperuricemia:
uric acid is the end product of purine metabolism
Normal concentration in serum is 2.5 -7mg / dl in men & 1.5-6mg/dl in women
Elevation in serum level referred as hyperuricemia
2. Gout:
• Metabolic diseases associated with over production of uric acid, where crystals of sodium urate gets deposited in soft tissue like joints. Such deposit is known as tophi and leads to gouty arthritis
• More common in men than in women
• Two types of gout:
• Primary gout: In born error of metabolism due to over production of uric acid (treatment by Allopurinol etc)
• Hyperuricemia of primary gout is due to excessive production of purines and to renal retention of uric acid
• Excessive purine synthesis is due to deficiency of hypoxanthine-guanine phosphoribosyl transferase
• Secondary Gout: Due to various diseases which causes increased synthesis or decreased excretion of uric acid
• Increased degradation of nucleic acids (hence more uric acid formation) is observed in various cancers (leukemias, polycythemia, lymphomas, etc.) psoriasis and increased tissue breakdown (trauma, starvation etc.)
• Disorders associated with impairment in renal function cause accumulation of uric acid which may lead to gout
Summary:
• Two major sources of nucleotides are salvage pathway and de novo biosynthesis
• Purine nucleotides are biodegraded by nucleotidases, nucleotide phosphorylases, deaminases & xanthine oxidase
• Uric acid is the final product of purine biodegradation in mammals
• Defective purine metabolism leads to clinical disease
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf