ANTIPSYCHOTICS AGENTS
Intended
learning outcomes
At the end of this
lecture, student will be able to:
• Define psychotropic drugs
• Categorize the psychotropic agents
• Describe the SAR of phenothiazines
• Discuss about some antipsychotic agents
• Outline the synthesis of some antipsychotic agents
Contents
• Psychotropic drugs
• Categorize the psychotropic agents
• The SAR of Phenothiazine
• Important drugs used as antipsychotic agents
• The synthesis of some antipsychotic agents
Psychotropic
agents/Tranquilizers
• Psychoactive or psychotropic drugs – also known as
tranquilizers
• These drugs are used in the treatment of psychiatric
disorders i.e. abnormalities of mental function
• The psychoactive drugs render the patient calm and
peaceful by reducing agitation and anxiety
• They cause sedation without inducing sleep
• Psychoactive drugs does not cure mental disorders, control
most symptomatic manifestations and behavioral deviances
• Tranquilizers are drugs essentially used in the management
and, treatment of psychoses and neuroses
• They specifically exert their action on the lower brain
areas to produce emotional calmness and relaxation without appreciable hypnosis
sedation euphoria or motor impairment
• In addition many of these drugs also display clinically
beneficial actions, for instance skeletal muscle relaxants, antihypertensive,
antiemetic and antiepileptic properties
• Major tranquilizers and minor tranquilizers have overlapping
characteristic features
• Anti-psychotic drugs are used to treat psychoses like
schizophrenia, mania, senile dementia and behavior disorders in children
• These drugs act by depressing the central nervous system
(by decreasing dopamine levels)
• They produce sedation without producing sleep
• Their overall influence is to free the mind from
disturbance and thus calm the mind
• They reduce excitation, agitation, aggressiveness and
impulsiveness
• Drugs which better mental aberrations that are invariably
characteristic feature of psychoses
Psychotropic
Agents- Classification
• The primary characteristic feature of these drugs is that
they alter the mental state and behavior in a predictable way.
• The psychoactive drugs are classified as;
1. Antipsychotic drugs – Major tranquilizers (for psychoses)
2. Anti-depressant drugs – Minor tranquilizers (for
neuroses)
3. Anti-anxiety drugs
Psychoses-Symptoms
• Positive symptoms
of psychoses essentially comprise of a host of disorders, such as: mild behavioral
changes anxiety, delusions, hallucinations, and schizophrenias.
• Negative symptoms
are usually designated by cognitive deficits, social withdrawal, apathy, and
anhedonia
Psychoses-
cardinal requirements
The typical ‘antipsychotics’ should ideally possess the
following cardinal requirements, such as:
• High lipid
solubility
• Affinity for
protein-binding (92–99%),
• Large volume of
distribution (vd 55) i.e., greater than 7 L.kg–1,
• Variance in oral
bioavailability (25–35%), and
• Short plasma
half-life between 10–20 hours
Classification of antipsychotic agents
The drugs used in the treatment of psychoses are classified
as follows:
1. Phenothiazine
derivatives: Promazine hydrochloride, Chlorpromazine
hydrochloride*,Triflupromazine, Thioridazine
hydrochloride, Piperacetazine hydrochloride, Prochlorperazine maleate,
Trifluoperazine hydrochloride
2. Fluro
butyrophenones: Haloperidol, Droperidol, Risperidone
3.
Dihydroindolones/Beta amino ketones: Molindone hydrochloride
4. Thioxanthines/Ring
Analogues of Phenothiazines: Chlorprothixene, Thiothixene, Loxapine
succinate, Clozapine
5. Benzamides:
Sulpieride
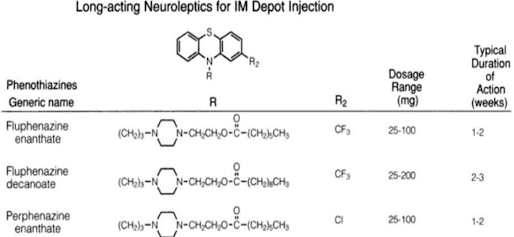
Phenothiazines
• Phenothiazines act exclusively on specific postsynaptic
receptors and block the postsynaptic dopamine receptors.
They work on the positive symptoms of psychosis such as
hallucinations, delusions, disorganized speech, looseness of association, and
bizarre behavior.
• The following is the general structure of phenothiazines
SAR OF
PHENOTHIAZINES
The neuroleptic properties of phenothiazine may be affected
by following:
• Nature of the chain in the position N10
• Nature of the amino group
• Substitution at second position
• Replacement of the H in position 2
• Substitution at position 3
• Substitution at position 1
• Three carbon chain
• Branching with larger groups
• Replacement of terminal alkly amino group
1. Unsubstituted Phenothiazines has no activity but has
enough lipophilicity for good brain penetration
• Substitution at C2 and N10 is required for activtiy
2. Potency increases in the following order of position of
ring substituents
1˂4˂3˂2
C2 must have an electron withdrawing group
The activity for these various group is as
X = – SO2NR2 > -CF3 > -CO-CH3 > -Cl
Ex: Chlorpromazine (chlorine)
3) A terminal amino substituent must be present at N10.
It can be piperazine, piperidine or aliphatic and their
intensity could be ranked as follows: piperazine group >piperidine group
> aliphatic chain
A piperidine ring in the side chain show lower incidence of
extrapyramidal side effects (e.g. Parkinsonism) possibly due to increased
central antimuscarinic activity. E.g. thioridazine.
• Esterification of the OH containing piperazine derivatives
extensively increases the duration of action, less hypotension
4. Alkyl Side chain:
There must be an linear (ie unbranched) alkyl linker between the core ring and
the terminal amino ring those length is optimum at three methylene units ie
CH2-CH2-CH2
Reduction of these carbon number changes receptor affinity
• Branching at the β position of the side chain with small
methyl group – DECREASE IN ACTIVITY.
• β position substitution with larger group – LOSS IN
ACTIVITY. Bridging of position 3 of the side chain to position 1 – LOSS IN
ACTIVITY
Phenothiazine ring:
• Oxidation of 5
sulphur: decrease activity
• 1,2,3,4 aza
phenothiazine: more potent
For example: 1-aza analogue of promazine is more potent than
parent compound
• If nitrogen is replaced by carbon then the compound
thioxanthine is produced which is also active but less potent compared to
phenothiazine
• Phenothiazines
exert their antipsychotic potency by interacting with a receptor at three
marked sites X, Y, Z to produce a singificant response.
– The order of specific structural requirement at these
sites is YZX. However, a three-carbon chain at site Y affords an optimal
antipsychotic activity
Promazine
Hydrochloride
• Promazine is a
phenothiazine derivative
• Chemically it
is 10-[3-(dimethyl amino)-propyl] phenothiazine
Properties
• Promazine is
available as hydrochloride salt. Promazine HCl is white or slightly yellow
crystalline powder, and is freely soluble in water and chloroform. It should be
protected from air.
Medicinal Uses
• Promazine has
antipsychotic properties: dopamine receptor antagonist and neuroleptic
• It is also used
to control nausea and vomiting
• An older
medication used to treat schizophrenia
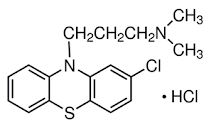
Chlorpromazine
hydrochloride INN, BAN, USAN
• Chlorpromazine
hydrochloride is a phenothiazine derivative and
• Chemically it
is 2-chloro-10-[3-(-dimethylamino) propyl] phenothiazine monohydrochloride
Properties
• Chlorpromazine
hydrochloride is an odorless, white crystalline powder. It is freely soluble in
water, alcohol, chloroform and insoluble in ether and benzene. It decomposes on
exposure to air and light hence it should be stored in airtight containers and
protect from light
Medicinal Uses
• Chlorpromazine
is used in the management of psychotic conditions. It also controls excitement,
aggression and agitation.
• It has
antiemetic, antipruritic, anti-histaminic and sedative properties
Chlorpromazine
hydrochloride Mechanism of Action:
• Exact mechanism
is not absolutely determined. Chlorpromazine is thought to block dopamine at D2
receptor sites in the mesolimbic medullary chemoreceptor trigger zone areas of
the brain.
• It causes
inhibitory post-synaptic effects by reducing the flow of dopamine as the
dopaminergic ion channels are closed
Chlorpromazine
hydrochloride Synthesis:
Step I:
• Chlorpromazine
is synthesized by cyclization of 3- chloro diphenylamine with sulphur in
presence of small amount of iodine as catalyst
Step II:
• Refluxing a
toluene solution of 2-chlorophenothiazine and 3-chloropropyl dimethylamine in
the presence of sodamide for several hours, followed by filtration and removal
of toluene under reduced pressure.
Triflupromazine
• Triflupromazine
is a fluorinated phenothiazine derivative.
• Chemically
triflupromazine is 10-[3-(dimethylamino)propyl]-2-(trifluoromethyl) phenothiazine
Properties:
• Triflupromazine
occurs as hydrochloride salt. Triflupromazine hydrochloride is white,
crystalline powder. It is freely soluble in water, alcohol and insoluble in
ether.
Medicinal Uses:
• Triflupromazine
is used to treat psychotic disorders
• It also has
antiemetic properties
Thioridazine
hydrochloride
Properties: It is
a white or almost white crystalline powder, soluble in ethanol, freely soluble
in water and in methanol.
Medicinal uses:
• The drug has
sedative and hypotensive activity in common with chlorpromazine.
• It is effective
in the management and manifestations of psychotic disorders, used as Dopamine
receptor antagonist and neuroleptic
• The drug exerts
minimum antiemetic activity and there by gives rise to minimal extrapyramidal
stimulation
Piperacetazine
hydrochloride
• Chemically it
is 1-(10-{3-[4-(2-Hydroxyethyl)-1-piperidinyl]propyl}-10H-phenothiazin-2-yl)ethanone
hydrochloride
• It is an
antipsychotic prodrug, most notably used for schizophrenia
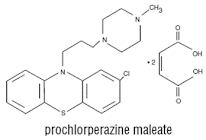
Prochlorperazine
maleate
Properties: It is
a white or pale yellow crystalline powder, well soluble in water and alcohol.
• It is a
dopamine (D2) receptor antagonist that belongs to the phenothiazine class of
antipsychotic agents
Medicinal uses:
• For the
antiemetic treatment of nausea and vertigo
• It is also a
highly potent typical antipsychotic, 10–20 times more potent than
chlorpromazine
• It is also used
to treat migraine headaches
• It is used to
prevent vomiting caused by chemotherapy, radiation therapy and in the pre- and
postoperative setting
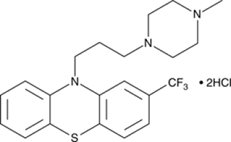
Trifluoperazine
hydrochloride.
• Chemically it
is 10-[3-(4-methyl-1-piperazinyl)propyl]-2-(trifluoromethyl)-10H-phenothiazine,
dihydrochloride
• Trifluoperazine
(TFP) is a phenothiazine compound with anti-adrenergic and anti-dopaminergic
actions typical of antipsychotic agents
Thioxanthines/Ring
Analogues of Phenothiazines:
Chlorprothixene
Thiothixene
Loxapine succinate
Clozapine
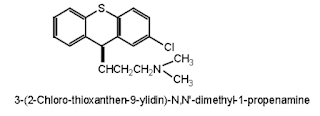
Chlorprothixene
• Chlorprothixene
is a typical antipsychotic drug of the thioxanthene (tricyclic) class.
• Chemically it
is (3Z)-3-(2-Chloro-9H-thioxanthen-9-ylidene)-N,N-dimethyl-1-propanamine
Properties: It is
a white or almost white crystalline powder, slightly soluble in methylene
chloride, and soluble in water and alcohol.
Medicinal uses
• It is used in
the treatment of acute and chronic schizophrenia, psychotic and other
conditions in which anxiety, agitation, and tension predominate.
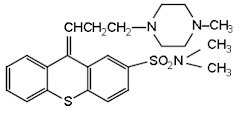
Thiothixene
• Thiothixene is
a thioxanthine used as an antipsychotic agent. Its effects are similar to the
phenothiazine antipsychotics.
• Chemically it is
(9Z)-N,N-dimethyl-9-[3-(4-methylpiperazin-1-
l)propylidene]thioxanthene-2-sulfonamide
Properties:
White, or nearly white crystalline powder with slight odour, and affected
bylight, soluble in water, anhydrous alcohol, or chloroform, practically
insoluble in benzene, acetone, orether. The substituent in the second position
produces Z and E isomers. The Z isomers are the moreactive antipsychotic
isomers.
Medicinal uses
• It was
introduced as an antipsychotic agent useful in the management of schizophrenia
and other psychotic states.
• It is also
helpful in the management of secondary symptoms of schizophrenia, such as
hallucinations, tension, and suspiciousness.
• It also shows
antidepressant property.
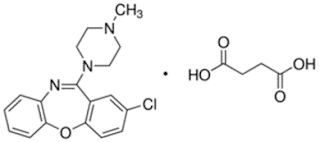
Loxapine
succinate
• Loxapine
succinate is an antipsychotic agent used in schizophrenia.
Medicinal uses:
• D2/D4 dopamine
receptor antagonist
• 5-HT2A/2B, 5-H7
serotonin receptor antagonist
• Dibenzoxazepine
antipsychotic agent
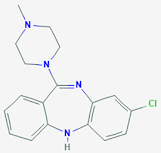
Clozapine
• Clozapine is a
tricylic dibenzodiazepine, classified as an atypical antipsychotic agent.
• It binds
several types of central nervous system receptors, and displays a unique
pharmacological profile.
• It may be
better than other antipsychotics in people with both schizophrenia and
Parkinson’s disease
• Chemically it
is 3-chloro-6-(4-methylpiperazin-1-yl)-11H-benzo[b][1,4]benzodiazepine
Fluro
butyrophenones:
Haloperidol
Droperidol
Risperidone
Fluro butyrophenones:
Haloperidol
INN, BAN, USAN:
• Haloperidol is
a butyrophenone
Properties: White to faintly yellowish, practically
insoluble in water, soluble in methanol-acetone, benzene,
Medicinal uses
• It is useful in
the management of psychotic reactions, hostility, and hyperactivity.
• It is a drug of
choice for Tourett’s syndrome.
• Haloperidol is
an effective neuroleptic
• Antiemetic
properties
Droperidol:
• Droperidol is
butyrophenone derivative used as sedative, tranquilizer, and anti-nausea
medicine
Properties and uses:
It is a white or almost white powder insoluble in water, sparingly soluble in
alcohol, freely soluble in dimethyl formamide and in methylene chloride.
Medicinal Uses:
• It is
frequently used in combination with the nacrotic agents pre-anaesthetically.
• It is a
neuroleptic used as an adjunct to anaesthesia to produce sedation and reduce
incidence of nausea and vomiting.
• Also used as
β1-adrenoceptor agonist α-adrenoceptor agonist
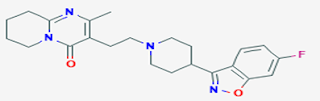
Risperidone:
• benzisoxazole
derivative or flurobutyrophenones
• Chemically it
is
3-[2-[4-(6-fluoro-1,2-benzoxazol-3-yl)piperidin-1-yl]ethyl]-2-methyl-6,7,8,9-tetrahydropyrido[1,2-a]pyrimidin-4-one
Properties:
• Risperidone is
a white to slightly beige powder.
• It is
practically insoluble in water, freely soluble in methylene chloride, and
soluble in methanol and 0.1 N HCl
Mechanism of action of Risperidone:
• It has been
proposed that the drug’s therapeutic activity in schizophrenia is mediated
through a combination of dopamine Type 2 (D2) and serotonin Type 2 (5HT2)
receptor antagonism
Medicinal uses:
• It is a
medication known as an atypical antipsychotic that is used to treat symptoms of
schizophrenia in teenagers and adults.
• Mood disorders,
including bipolar disorder and depression with pyschosis
Beta amino
ketones/Dihydro indolones:
Molindone
hydrochloride
Properties:
Exists as white crystals, freely soluble in water or alcohol,
Medicinal uses:
• It is a potent
antipsychotic as trifluoperazine and all the side effects resemble those of the
phenothiazines.
• It is used in
the treatment of schizophrenia and other psychosis
Benzamides:
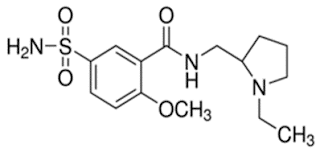
Sulpieride
• Sulpiride is a
substituted benzamide derivative
• Chemically it
is N-[(1-ethylpyrrolidin-2-yl)methyl]-2-methoxy-5-sulfamoylbenzamide
• A dopamine
D2-receptor antagonist
• It has been
used therapeutically as an antidepressant, antipsychotic, and as a digestive
aid.
Summary
• These drugs are used in the treatment of psychiatric disorders
i.e. abnormalities of mental function.
• The psychoactive drugs render the patient calm and
peaceful by reducing agitation and anxiety
• Phenothiazines exert their antipsychotic potency by
interacting with a receptor at three sites to produce a singificant response