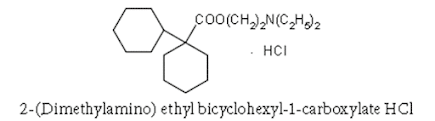Cholinergic Blocking Agents or Anticholinergic Drugs

Intended learning outcomes
At the end of the lecture students will be able to
• Cholinergic Blocking agents: SAR of cholinolytic agents
• Solanaceous alkaloids and analogues
• Outline the synthesis of Ipratropium bromide
• Synthetic cholinergic blocking agents
• Outline the synthesis of Dicyclomine hydrochloride and Procyclidine hydrochloride
The parasympatholytics or cholinergic blocking agents:
• The parasympatholytics or cholinergic blocking agents or antimuscarinic include atropine and related alkaloids obtained from plants, such as Atropa belladona, A.accuminata, Hyosyamus niger, Scoplola carniolica, Datura strammonium, and synthetic or semisynthetic atropine substitutes.
• These drugs in the therapeutic doses predominantly block the muscarinic actions of acetylcholine, but the ganglionic and skeletal neuromuscular actions of acetylcholine are not affected
• The term ‘antimuscarinic’ is derived from the action of acetylcholine at the postganglionic synapse which is closely imitated by the alkaloid, muscarine.
• Antimuscarinic agents like atropine chiefly exert their action by blocking the normal responses to excitation of postganglionic parasympathetic (cholinergic) nerves that stimulate both smooth muscle as well as exocrine glands.
• Sometimes they are also termed as parasympatholytic and as anticholinergic agents.
• They usually act by preventing the normal effect of acetylcholine on the receptor cells
Mechanism action of Antimuscarinic agents:
• The pharmacological effects are blockage of parasympathetic stimulation at effector organs.
• They are rapidly absorbed from the gastrointestinal tract, slowly absorbed when applied locally on eye or skin
• The antimuscarinic effects mainly include an elevation of heart- rate. Besides, a diminution in the production of bronchial, lachrymal, gastric, nasal, intestinal, sweat and saliva secretions are observed together with a reduction in intestinal motility
Classification of cholinergic blocking agents :
The currently available classes of drugs include the following:
1. Naturally occurring alkaloid- atropine and scopolamine (atropine from a plant A. belladona).
2. Semisynthetic derivatives such as Homatropine, Atropine methonitrate, Ipratropium bromide, and Tiotropium bromide.
3. Synthetic congeners (show some selectivity for nicotinic and muscarinic receptors).
Out of these synthetic compounds, according to their various therapeutic uses, they have been segregated into mydriatics (cyclopentolate), antisecretory, and antispasmodics (propantheline, oxyphenonium, dicyclomine, and pirenzepine) and also antiparkinson’s drugs (trihexyphenidyl, procyclidine, and biperiden).
Cholinergic Blocking agents SAR:
• Anticholinergic agents are bulky.
• They combine with muscarinic receptors and shield the binding site from acetylcholine.
• The general structure of the compounds in this category is as shown above
• Substituent R1 should be carbocyclic or heterocyclic ring for maximal antagonist activity
• Substituent R2 should be a hydrogen atom, hydroxy group, hydroxymethyl group, or methyl group
• The nature of the group X effects only the duration of action, the physicochemical properties and the side effects of the drug molecule but not its ability to bind with the receptor
• There is a limitation for the N-substitution. Optimal potency is associated with 2-3 ethyl groups
• The stereochemistry at the benzylic carbon is critical for muscarinic antagonist activity.
• Any compound that can place the phenyl group in the same absolute configuration as depicted in the general formula above will have potent muscarinic antagonist activity
• The phenyl ring cannot tolerate any their substituent than F at the p- position without losing its antagonist activity
• A negative site for binding of the positive charged N; quaternary amines have formal positive charge while tertiary amines have a positive charged proton
• Atropine is a racemic mixture (equal number of d- and l-isomers) and like most chemicals acting on the peripheral nervous system, atropine is stereospecific; l-isomer (l-hyoscyamine) is 250 times more active than the d-isomer
• The presence of an N-methyl group on atropine or scopolamine changes the activity of the ligand, possibly by preventing a close interaction between the ligand and the membrane or lipophilic sites on the receptor. The methyl group also prevents the penetration into
Therapeutic uses of anticholinergic drugs
1. Peripheral: Therapeutic uses following inhibition of parasympathetic transmission, e.g., mydriasis with cycloplegia, decrease saliva production, decrease motility of smooth muscle, inhibition of vagal transmission to heart, decrease bronchial secretions, decrease in urinary incontinence, etc.
2. Central: Anti-parkinson and anti-motion sickness
3. If anticholinergic drugs are non-quaternary amine derivatives, they will cross the blood brain barrier. They may have therapeutic actions, or side effects, involving the central nervous system; if anticholinergic drugs are quaternary amines, they will not cross the blood brain barrier, thus they are devoid of CNS activity.
4. Expected ‘side effects’ of anticholinergic therapy include: peripheral-photophobia, cycloplegia, dry mouth, tachycardia, difficult urination, red skin (‘atropine flush’), and increase in skin temperature, central-sedation or excitement.
A. Solanaceous alkaloids and analogues
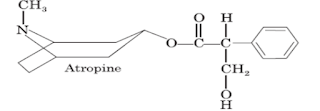
i. Atropine (Atp, Tropine)
Chemical Structure of Atropin
Properties:
• It is a white crystalline powder or colourless crystals, freely soluble in alcohol and well soluble in water.
• It is the tropine ester of racemic tropic acid and is optically inactive
Medicinal uses:
• Antidote for acetyl choline
• Antagonist of muscarinic cholinergic receptors
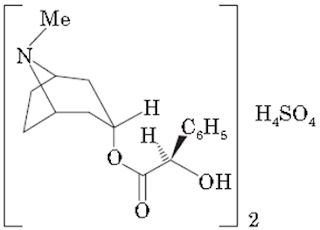
ii. Hyoscyamine sulphate
Properties:
• Hyoscyamine sulphate is available as white, crystalline powder or colorless needles, very soluble in water, sparingly soluble or soluble in alcohol, practically insoluble in ether. It melts at about 203°C, with decomposition. It should be stored in an airtight container, protected from light.
• It is a salt of a belladonna alkaloid derivative
Chemical Structure of Hyoscyamine sulphate
Medicinal uses:
• An anticholinergic drug used to treat peptic ulcers
iii. Scopolamine hydrobromide:
Chemical Structure of Scopolamine hydrobromide
It is a salt form of scopolamine, a tropane alkaloid derived from plants of the nightshade family (Solanaceae), specifically Hyoscyamus niger and Atropa belladonna
Properties: It exists as colourless or white crystals or white granular powder, odourless, slightly efflorescent in dry air, and is an anhydrous salt, soluble in water or alcohol and in chloroform, insoluble inether. Scopolamine is the levo component of the racemic mixture that is known as Hyoscine.
Medicinal uses:
• Useful in preventing nausea and vomiting, especially due to motion sickness.
• Scopolamine has antispasmodic activity
iv. Homatropine hydrobromide:
Chemical Structure of Homatropine hydrobromide
• It is the hydrobromide salt form of homatropine, a synthetic tertiary amine alkaloid with antimuscarinic properties.
• Homatropine, a competitive inhibitor of acetylcholine at the muscarinic receptor, blocks parasympathetic nerve stimulation
Properties: It is a white crystalline powder or colourless crystals, sparingly soluble in alcohol, but freely soluble in water.
Medicinal uses:
• To treat peptic ulcer and gastro-intestinal spasm
• It is used topically on the ciliary structure of the eye and to effect mydriasis (dilation of the pupil of the eye)
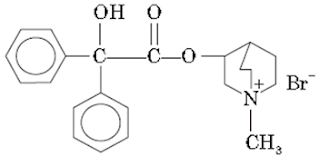
v. Ipratropium bromide:
• Ipratropium Bromide is the bromide salt form of ipratropium, a synthetic derivative of the alkaloid atropine with anticholinergic properties.
• Ipratropium antagonizes the actions of acetylcholine at parasympathetic, postganglionic, effector-cell junctions.
• When inhaled, ipratropium binds competitively to cholinergic receptors in the bronchial smooth muscle thereby blocking the bronchoconstrictor actions of the acetylcholine
Chemical Structure of Ipratropium bromide
Properties: It is a white or almost white crystalline powder, freely soluble in methanol, soluble in water, but slightly soluble in ethanol.
Medicinal uses:
• It is used in the inhalation therapy to produce dilation of bronchial smooth muscle for acute asthmatic attacks
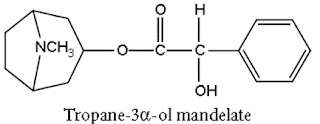
Synthesis of Atropine
Synthesis of Ipratropium bromide
B. Synthetic cholinergic blocking agents:
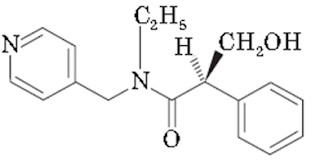
i. Tropicamide:
Chemical Structure of Tropicamide
• Tropicamide is a synthetic muscarinic antagonist with actions similar to atropine and with an anticholinergic property.
• Tropicamide is (RS)-N-ethyl-2-phenyl-N-(4-pyridylmethyl) hydrocrylamide. Tropicamide is a member of acetamides.
• This inhibits the responses from cholinergic stimulation, producing dilation of the pupil and paralysis of the ciliary muscle.
Properties:
• It is white, crystalline powder, slightly soluble in water, freely soluble in alcohol and in methylene chloride.
Medicinal uses:
• It is an effective anticholinergic drug for ophthalmic use.
• Diagnostic agent and is used to produce short-duration mydriasis and cycloplegia
ii. Cyclopentolate hydrochloride
• Cyclopentolate hydrochloride is the hydrochloride salt of cyclopentolate
• It is also an aminoalcohol ester.
• Cyclopentolate hydrochloride is 2-(dimethylamino)-ethyl-1-hydroxy-α-phenylcyclo pentane acetate hydrochloride.
Chemical Structure of Cyclopentolate hydrochloride
Properties:
It is available as colorless, odorless, crystalline powder.
It is soluble in water and in alcohol.
Medicinal uses:
• Cyclopentolate hydrochloride is used as a mydriatic agent
• It is used for the treatment of iritis, keratitis, and choroiditis
iii. Clidinium bromide
• Clidinium is another aminoalcohol ester available as bromide
• It derives from a benzilic acid and a 3-quinuclidinol.
• Clindinium bromide is 3-hydroxy-1-methylquinuclidinium bromide
Chemical Structure of Clidinium bromide
Properties:
It is available as colorless odorless, crystalline powder.
It is an optically active compound. It is soluble in water, and alcohol.
Medicinal uses:
• Clidinium bromide is an anticholinergic agent
• It is used for the treatment of peptic ulcer, hyperchlorhydria, and ulcerative or spastic colon
• An antispasmodic drug and an anti-arrhythmia drug
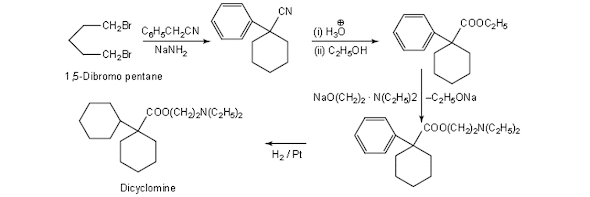
iv. Dicyclomine hydrochloride
Chemical Structure of Dicyclomine hydrochloride
• Dicyclomine Hydrochloride is the hydrochloride salt form of dicyclomine, a synthetic analog of acetylcholine with antimuscarinic activity.
• Dicyclomine hydrochloride antagonizes muscarinic receptors on smooth muscle in the gastrointestinal (GI) tract, thereby preventing the actions of acetylcholine and reducing GI smooth muscle spasms.
• Dicyclomine hydrochloride is 2-(diethylamino) ethyl bicyclohexyl-1- carboxylate hydrochloride
Properties:
• A white or almost white, crystalline powder, soluble in water, freely soluble in alcohol and in methylene chloride. It shows polymorphism.
Medicinal uses:
Muscarinic antagonist used as an antispasmodic and in urinary incontinence
Dicyclomine Synthesis
v. Glycopyrrolate
Chemical Structure of Glycopyrrolate
• Glycopyrrolate is an anticholinergic agent used to treat gastrointestinal conditions associated with intestinal spasm and to decrease secretions during anesthesia
• Chemically glycopyrrolate is 3-hydroxy-1, 1-dimethylpyrrolidinium bromide α-cyclopentylmandelate
Properties:
• It is available as white, crystalline powder. Glycopyrrolate is soluble in water and alcohol but insoluble in chloroform and ether. It should be stored in a tightly sealed container away from heat and direct light.
Medicinal uses:
• Anticholinergic agent with antispasmodic activity.
vi. Methantheline bromide
Chemical Structure of Methantheline bromide
• A quaternary ammonium compound that acts as an antimuscarinic agent.
Properties: A white powder
Medicinal uses:
• It has been used in the treatment of PEPTIC ULCER, in gastrointestinal disorders associated with smooth muscle spasm
• Used in the management of urinary incontinence
• Used for the treatment of HYPERHIDROSIS
vii. Propantheline bromide
Chemical Structure of Propantheline bromide
It is the bromide salt form of propantheline, a quaternary ammonium compound structurally related to belladonna alkaloids
Properties:
• It is a white or yellowish-white powder, slightly hygroscopic, soluble in water, in alcohol and in methylene chloride.
Medicinal uses:
• It is benefiial for the treatment of peptic ulcer, due to the decreased gastric motility by this drug, and it may relieve the pain in this condition.
• It is used as antispasmodic
viii. Benztropine mesylate
Chemical Structure of Benztropine mesylate
• Benztropine mesylate is an aminoalcoholether. It is (1R, 3R, 5S) –3- benzhydryloxytropane methanesulphonate
• It is a synthetic antiparkinson tertiary amine structurally related to atropine,
• Benztropine Mesylate acts as a central muscarinic antagonist and inhibits dopamine uptake.
Properties:
• It is available as a white, crystalline powder, odourless. Very soluble in water, freely soluble in ethanol, practically insoluble in ether.
Medicinal uses:
• Anticholinergic, antihistaminic and local anaesthetic
• It is also used in the treatment of Parkison’s disease
ix. Orphenadrine citrate
Chemical Structure of Orphenadrine citrate
• Orphenadrine Citrate is the citrate salt form of orphenadrine with a muscle relaxant property
• Orphenadrine citrate is (RS)—N, N-dimethyl-[2-(o-methyl-α- phenylbenzyloxy) ethyl amine citrate.
Properties:
• It is available as a white or almost white, odorless, crystalline powder.
• Sparingly soluble in water, slightly soluble in ethanol, practically insoluble in chloroform and in ether.
Medicinal uses:
• It has anticholinergic and antihistaminic activities
x. Biperidine hydrochloride
Chemical Structure of Biperidine hydrochloride
• Biperiden is an oral anticholinergic agent used predominantly in the symptomatic therapy of Parkinson disease and movement disorders
Properties:
• Biperiden hydrochloride is white, crystalline powder, slightly soluble in water and in alcohol, very slightly soluble in methylene chloride, practically insoluble in ether. It melts at about 280°C with decomposition.
Medicinal uses:
• To treat parkinson’s disease
• It is also used to cure akineasia, rigidity and tremor
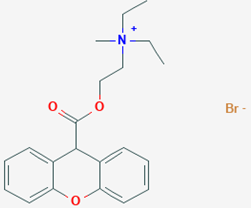
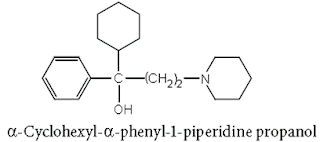
xi. Procyclidine hydrochloride
Chemical Structure of Procyclidine hydrochloride
• Procyclidine Hydrochloride is the hydrochloride salt form of procyclidine, with anticholinergic properties
Properties:
• It exists as white crystalline powder, and it has been used for peripheral effects that are similar to methantheline.
Medicinal uses:
• To treat parkinson’s disease
Procyclidine hydrochloride Synthesis
xii. Tridihexethyl chloride
Chemical Structure of Tridihexethyl chloride
• Tridihexethyl chloride is an organic chloride salt. It contains a tridihexethyl.
Properties:
• It is a white crystalline powder, slightly soluble in water, sparingly soluble in alcohol and in methylene chloride.
Medicinal uses:
• It is used as antispasmodic and antiparkinsonian agent.
• Trihexylphenidyl is more effective than levodopa against Parkinson’s tremor
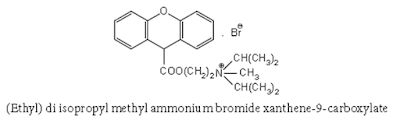
xiii. Isopropamide iodide
Chemical Structure of Isopropamide iodide
• Isopropamide is an aminoamide and occurs as ispropamide iodide.
• Chemically isopropamide is (3-carbamoyl-3, 3-diphenyl propyl) diisopropylmethyl ammonium iodide.
• Isopropamide iodide is a diarylmethane.
Properties:
• Isopropamide iodide is pale yellow coloured, bitter taste crystalline powder. It is sparingly soluble in water and freely soluble in chloroform and alcohol.
Medicinal uses:
• Isopropamide is a potent anticholinergic drug.
• It has antispasmodic and antisecretory effects.
• It is used in the treatment of peptic ulcer.
xiv. Ethopropazine hydrochloride/Profenamine hydrochloride
Chemical Structure of Ethopropazine hydrochloride/Profenamine hydrochloride
• It is the monohydrochloride salt of profenamine.
Medicinal uses:
• An antimuscarinic
• It is used for the symptomatic treatment of Parkinson’s disease.
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf