Para sympathomimetic agents
Intended learning outcomes
At the end of the lecture students will be able to
• SAR of Para
sympathomimetic agents
• Classify cholinergic
drugs
• Chemical structure,
medicinal uses of classified cholinergic drugs
• Outline the synthesis
of Carbachol
• Write chemical structure,
medicinal uses of Indirect acting/ Cholinesterase inhibitors (Reversible &
Irreversible)
• Outline the synthesis
of Neostigmine
• Cholinesterase
reactivator: Pralidoxime chloride
Contents
• SAR of Para
sympathomimetic agents
• Classify cholinergic
drugs
• Drug profile of
cholinergic drugs
• Outline the synthesis
of Carbachol
• Drug profile of indirect
acting/ Cholinesterase inhibitors (Reversible & Irreversible)
• Outline the synthesis
of Neostigmine
• Cholinesterase reactivator:
Pralidoxime chloride
SAR of Para sympathomimetic agents
Onium Group
• Essential for affinity
and intrinsic activity
• It interact with the –vely
charged Aspartic acid residue of the receptor
• Trimethylammonium group
is optimal function moiety. (Exception is pilocarpine, arecoline, nicotine)
• Substitution with
larger alkyl groups decrease the activity.
Ester Group
• Essential for affinity,
forms H bond with threonine and asparagine residue at the receptor site.
• When methyl replaced by
higher homologues (i.e., the propionyl group), the resulting esters are less
potent the Ach.
• Aromatic Group possess
cholinergic antagonist activity
• NH2 Group (carbamic
acid ester group)
• It is more stable than
carboxylate esters to hydrolysis.
Ethylene Bridge
• Shortening or
lengthening of ethylene bridge decrease M activity
• α substation decrease both M (in greater
extent) and N Activity.
• β substation decrease both M (in greater extent)
and N Activity.
Classification
of Para sympathomimetic agents
Para sympathomimetic agents
Direct-acting cholinergic
drugs:
There are two classes of
the drugs:
(A) Choline esters and
(B) Cholinomimetic
alkaloids
Mechanism of Action of Directly Acting Cholinergic Drugs
• These drugs mediate the
actions through muscarinic and nicotinic receptor subtypes.
• Stimulation of M1orM3
receptors causes hydrolysis of polyphosphoinositides and mobilization of
intracellular Ca2+, as a consequence of interaction with a G protein and phospholipase
C is activated, which phosphorylates the target protein.
• In contrast, M2 and M4 inhibit
adenylcyclase and regulate specific ion channels, that is, enhancement of K+
conductance in cardiac arterial tissue.
• Cholinergic stimulation
affects cardiac function directly by inhibiting the effects of adrenergic
activation.
• As a part, it decreases
the cAMP formation and reduction on L-Type Ca2+ channel activity. In arterial
muscles, acetylcholine decreases the strength of contraction.
• This effect is due to
M2 receptor mediated action of G protein regulated K+ channels. Increased K+
permeability leads to hyperpolarization and shortens the duration of action
potentials.
Para sympathomimetic agents
Direct-acting cholinergic drugs
i. Acetylcholine chloride (Miochol)
Properties:
• It is a white or almost
white crystalline powder or colourless crystals, very hygroscopic in nature,
slightly soluble in methylene chloride, soluble in water and alcohol.
Medicinal uses:
• It is a topical ophthalmic
drug to induce miosis, during certain intraocular surgical procedures, such as
cataract surgery, ridectomy, penetrating keratoplasty, and other
anterior-segment surgery.
• Systemically
administered Ach is rapidly hydrolyzed by acetylcholinesterase, hence, it has
no clinical use.
• It is a cardiac
depressant and effective vasodilator.
ii. Methacholine chloride (Provocholine)
Properties: It is highly deliquescent, has faintly shy odour, and aqueous
solutions are neutral, soluble in water, alcohol, and CHCl3.
Medicinal uses:
• It is used to treat
Reynaud’s syndrome and glaucoma
• Methacholine was used in
the past to control supraventricular tachycardia and is replaced with
ectrophonium and other drugs, which are safer
• Methacholine is also
used for diagnosis of belladonna (i.e., muscarinic antagonist) poisoning
• For diagnosis of familial
dysautonomia, and for diagnosis of bronchial hyper reactivity (i.e.,
supersensitivity to bronchoconstriction in patients with asthma)
Cholinomimetic Alkaloids:
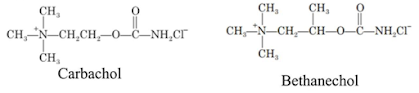
i. Carbachol
Properties:
• It is a white
crystalline, hygroscopic powder, soluble in water, sparingly soluble in
alcohol, insoluble in acetone.
• It is an ester of
carbamic acid, the terminal methyl group of Ach is replaced by amino group.
Medicinal uses:
• It possesses both
muscarinic and nicotinic properties by cholinergic receptor stimulation
• It is more slowly
hydrolyzed by acetylcholinesterase
• It is used for its
miotic actions in the treatment of glaucoma to reduce intraocular pressure
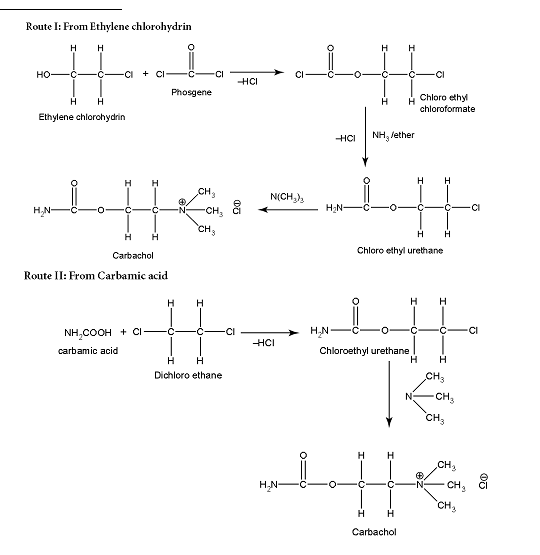
Carbachol Synthesis
ii. Bethanechol chloride
(Synonym: Urecholine, Myotonachol, Bethacol, Urotonin)
Properties: It is a white crystalline hygroscopic powder, and it
exhibits polymorphism, soluble in water and alcohol.
Medicinal uses:
• It has pharmacological
properties similar to those of methacholine.
The presence
of –CH3 gives
prolonged activity due
to steric hindrance.
• It produces smooth
muscle contractions.
• It can be given
subcutaneously, but not by intramuscular (IM) or intravenous (IV) because of
its severe side effects.
• It is used to relieve
urinary retention and abdominal distention after surgery. This is one of the
postvagotomy gastric drug
iii. Pilocarpine
Properties and uses:
• It is a white or almost
white crystalline powder or colourless crystals, hygroscopic,very soluble in
water and in alcohol.
• Pilocarpine is an alkaloid
obtained from the dried leaflets of Pilocarpus jaborandi and Pilocarpus
microphyllus in which it occurs to the extent of about 0.5% together with other
alkaloids.
• Chemically it is
3-ethyldihydro-4[(1-methyl-1H-imidazol-5-yl)- methyl] furan-2(3H)-one
• Pilocarpine is a
nonselective agonist on the muscarinic receptors.
• It acts on M3 receptors
in smooth muscles and cause contractions in the gut, trachea, and eyes.
• It is used for the
treatment of symptoms of dry mouth caused by radiotherapy for cancer of head and
neck and the symptoms associated with Sjogren’s syndrome.
• Mostly used as a
solution (1 to 5%) to exert an action on the eye to cause miosis and retard intraocular
tension in the treatment of open-angle glaucoma
Indirect Acting Cholinomimetic Drugs
• The actions of
acetylcholine released from autonomic and somatic motor nerves are terminated by
enzymatic destruction of the molecule
• Hydrolysis is
accomplished by acetylcholinesterase
• The indirect acting
drugs have primary effect on the active site of this enzyme, although some also
have direct actions at nicotinic receptors
• The common differences
between members of the group are chemical and pharmacokinetic, but their pharmacodynamics
properties are identical
Mechanism of Action of Indirectly Acting Cholinergic Drugs
(Anticholinesterase Agents)
• Acetylcholinesterase
(AchE) is a serine dependent isoenzyme capable of hydrolyzing Ach to choline
and acetic acid.
• The active site of AchE
comprises two distinct regions, an anionic site that possess a glutamate
residue and an esteratic site in which histidine imidazole ring and serine –OH
group are present.
• Catalytic hydrolysis
occurs, thereby the acetyl group is transferred to the serine –OH group,
leaving an acetylated enzyme molecule and a molecule of free choline
• Spontaneous hydrolysis
of the serine acetyl group occurs rapidly
There are two main categories of AchE inhibitors:
1. The amine or ammonium
AchE inhibitors react reversibly with enzymes, these compounds reversibly
acylate the esteratic serine hydroxyl, their duration of action are few minutes
to few hours.
2. The organophosphate
type AchE inhibitors form an irreversible fi rm bond with the enzymes
(esteratic site) and their duration of action are few weeks to months.
A. Reversible blockers
i. Physostigmine (Isopto-Eserine):
Properties:
• It exists as a white or
almost white crystalline powder, hygroscopic, very soluble in water, and freely
soluble in alcohol. It gradually becomes red when exposed to air and light; the
colour develops more quickly when the substance is also exposed to moisture.
• Aqueous solutions are
unstable. It melts at about 145°C with decomposition.
• It is an alkaloid
obtained from the dried ripe seeds of Physostigma venenosum.
Medicinal uses:
• Physostigmine is an
oldest anticholinesterase agent
• It is used in the
treatment of glaucoma
• It can penetrate the
blood brain barrier and is employed to antagonize the toxic CNS effects of
antimuscarinic drugs, tricyclic depressants, H1 antihistamines, and
benzodiazepines
• It is also used in the
treatment of Alzheimer’s disease
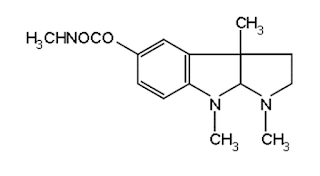
ii. Neostigmine Bromide
(Synonym: Prostigmine, Myostigmin, Tilstigmin)
Properties: It exists as white, odourless, crystalline powder with
a bitter taste, freely soluble in water, alcohol, and insoluble in ether. Its solutions
are neutral to litmus.
Medicinal uses:
It acts as a
cholinesterase inhibitor.
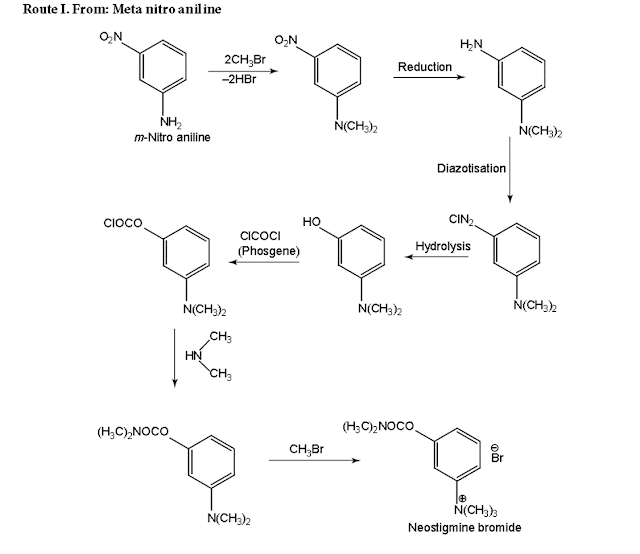
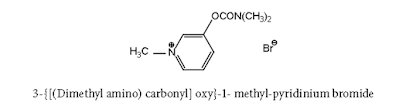
Neostigmine Bromide synthesis
iii. Pyridostigmine Bromide
(Synonym: Mestion, Pyrido, Trostigmin)
Properties: It exists as white, crystalline powder with a
characteristic odour and bitter taste, soluble in water, alcohol, chloroform,
slightly soluble in hexane, and insoluble in ether. It is hygroscopic in
nature.
Medicinal uses:
It is used in the
treatment of myasthenia gravis and it antagonizes the effects of neuromuscular
blocking (NMB) agents
iv. Edrophonium Chloride (Tensilon)
Properties: It exists as a white crystalline powder, soluble in
water and alcohol, insoluble in methylene chloride. On parenteral
administration, edrophonium has a more rapid onset and shorter duration of
action than neostigmine, pyridostigmine, or ambenonium.
Mechanism of action of Edrophonium Chloride:
Quaternary ammonium
compounds inhibit the enzyme reversibly by either binding with the esteratic
site, or with a site spatially removed, termed the peripheral anionic site
Medicinal uses:
• It is used as an
antiarrhythmic drug in paroxysmal atrial tachycardia.
• It is also used in the
diagnosis of myasthenia gravis
v. Tacrine Hydrochloride
• is the hydrochloride
salt form of tacrine, an aminoacridine derivative
Medicinal uses:
• Tacrine has been used
to counter the effects of muscle relaxants
• As a respiratory
stimulant
• In the treatment of
Alzheimer’s disease
vi. Ambenonium Hydrochloride
• Ambenonium is a bisquaternary
ammonium alcohol with parasympathomimetic activity
• It acts by suppressing
the activity of acetylcholinesterase
Chemically it is [oxalylbis(iminoethylene)]
bis[(ochlorobenzyl)diethylammonium]dichloride.
Properties: It is white, odourless, water soluble solid
Medicinal Uses: Cholinesterase Inhibitor
• Ambenonium is used to
treat myasthenia gravis.
B. Irreversible inhibitors
i. Echothiopate
Echothiopate is an
organophosphate available as echothiopate iodide.
Properties:
• Echothiopate iodide is
white, crystalline characteristic odor, (mercaptan like odous) hygroscopic
powder. It is soluble in water.
Medicinal Uses:
• Echothiopate is a long
acting irreversible anti-AChE drug that is used in the treatment of glaucoma.
ii. Malathion
• Malathion is another
effective pesticide, which is more effective on insects than on humans because
it requires biotransformation to the phosphate form, which can only be carried
out by insects..
• Chemically it is 2-
[(dimethoxyphosphinothioyl)thio]-butanedioic acid diethyl ester. Malathion is a
phosphodithioate ester
Properties: Malathion is available as a light amber colored liquid having
sulphur like odour
Medicinal uses:
• It is poor irreversible
inhibitor of choline esterase enzyme
• Malathion is used
extensively for controlling insects on vegetables, fruits, and cereal crops.
• It is also used for
controlling insects affecting man and animals
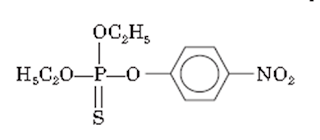
iii. Parathion
• Parathion is O,
O-diethyl O-p-nitrophenyl phosphorothioate.
• It is a weak
cholinesterase inhibitor.
Properties:
• Parathion occurs as a
brown colored liquid
Medicinal uses:
• Parathion is used as an
agricultural insecticide. It is especially used for controlling aphids, spider
mites, and scale insects
iv. Isofluorphate
• Isofluorphate is an
organophosphate. Isofluorphate covalently binds to acetylcholinesterase and
inhibits acetyl cholinesterase irreversibly.
Properties:
• Isofluorphate is a
colorless, water-miscible liquid.
Medicinal Uses:
• Isoflurophate is used
to treat glaucoma.
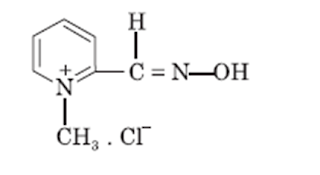
Cholinesterase reactivator:
Pralidoxime chloride:
• Pralidoxime is an
aldoxime and available as pralidoxime chloride.
• Chemically it is
2-formyl-1-methylpyridinium chloride oxime
Properties:
• Pralidoxime chloride is
colorless or light yellow colored, water soluble, and crystalline powder.
Medicinal Uses:
• The molecule
pralidoxime is a useful antidote for intoxication with cholinesterase
inhibitors such as the organophosphates.
• The molecule removes
the inhibitor from the active site in the form of an oxime phosphonate