DRUG METABOLISM

Intended learning outcomes
At the end of this lecture, student will be able to:
• Describe the drug metabolism.
• Explain the sites involved in biotransformation.
• List out the enzymes involved in drug metabolism
• Describe Phase I reactions and enzymes involved in first phase.
• Describe the phase II reactions
METABOLISM OR BIOTRANSFORMATION
• Metabolism is an essential pharmacokinetic process, which renders lipid soluble and non-polar compounds to water soluble and polar compounds so that they are excreted by various processes.
• The term metabolism is commonly used probably because products of drug transformation are called metabolites.
• This is because only water-soluble substances undergo excretion, whereas lipid soluble substances are passively reabsorbed from renal or extra renal excretory sites into the blood by virtue of their lipophilicity.
• Metabolism is a necessary biological process that limits the life of a substance in the body.
• Biotransformation: It is a specific term used for chemical transformation of xenobiotics in the body/living organism.
A series of enzyme-catalyzed processes—that alters the physiochemical properties of foreign chemicals (drug / xenobiotics) from those that favor absorption across biological membranes (lipophilicity) to those favoring elimination in urine or bile (hydrophilicity)
• Metabolism: It is a general term used for chemical transformation of xenobiotics and endogenous nutrients (e.g., proteins, carbohydrates and fats) within or outside the body.
• Xenobiotics: These are all chemical substances that are not nutrient for body (foreign to body) and which enter the body through ingestion, inhalation or dermal exposure.
They include: drugs, industrial chemicals, pesticides, pollutants, plant and animal toxins, etc.
Functions of Biotransformation
• It causes conversion of an active drug to inactive or less active metabolite(s) called as pharmacological inactivation.
• It causes conversion of an active to more active metabolite(s) called as bioactivation.
• It causes conversion of an inactive to more active toxic metabolite(s) called as lethal synthesis.
• It causes conversion of an inactive drug (pro-drug) to active metabolite(s) called as pharmacological activation
• It causes conversion of an active drug to equally active metabolite(s) (no change in pharmacological activity)
• It causes conversion of an active drug to active metabolite(s) having entirely different pharmacological activity (change in pharmacological activity)
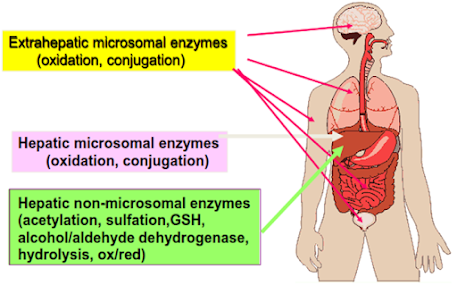
Site/Organs of drug metabolism
The major site of drug metabolism is the liver (microsomal enzyme systems of hepatocytes)
Secondary organs of biotransformation
• Kidney (proximal tubule)
• Lungs (type II cells)
• skin (epithelial cells)
• Intestines
• Plasma
• Nervous tissue (brain)
• Testes (Sertoli cells)
Liver
• The primary site for metabolism of almost all drugs because it is relatively rich in a large variety of metabolising enzymes.
• Metabolism by organs other than liver (called as extra-hepatic metabolism) is of lesser importance because lower level of metabolising enzymes is present in such tissues.
• Within a given cell, most drug metabolising activity is found in the smooth endoplasmic reticulum and the cytosol.
• Drug metabolism can also occur in mitochondria, nuclear envelope and plasma membrane.
• A few drugs are also metabolised by non-enzymatic means called as non-enzymatic metabolism.
• For example, atracurium, a neuromuscular blocking drug, is inactivated in plasma by spontaneous non-enzymatic degradation (Hoffman elimination) in addition to that by pseudocholine esterase enzyme.
Subcellular locations of metabolising enzymes
ENDOPLASMIC RETICULUM (microsomes): the primary location for the metabolizing enzymes.
(a) Phase I: cytochrome P450, flavin-containing monooxygenase, aldehyde oxidase, carboxylesterase, epoxide hydrolase, prostaglandin synthase, esterase.
(b) Phase II uridine diphosphate-glucuronosyltransferase, glutathione S-transferase, amino acid conjugating enzymes.
CYTOSOL (the soluble fraction of the cytoplasm): many water-soluble enzymes.
(a) Phase I: alcohol dehydrogenase, aldehyde reductase, aldehyde dehydrogenase, epoxide hydrolase, esterase.
(b) Phase II: sulfotransferase, glutathione S-transferase, N-acetyl transferase, catechol 0-methyl transferase, amino acid conjugating enzymes.
MITOCHONDRIA
Phase I: monoamine oxidase, aldehyde dehydrogenase, cytochrome P450.
Phase II: N-acetyl transferase, amino acid conjugating enzymes.
LYSOSOMES
Phase I: peptidase.
NUCLEUS
Phase II: uridine diphosphate-glucuronosyl transferase (nuclear membrane of enterocytes).
Drug Metabolising Enzymes
• A number of enzymes in animals are capable of metabolising drugs. These enzymes are located mainly in the liver, but may also be present in other organs like lungs, kidneys, intestine, brain, plasma, etc.
• The drug metabolising enzymes can be broadly divided into two groups: microsomal and non-microsomal enzymes.
• Microsomal enzymes: The endoplasmic reticulum (especially smooth endoplasmic reticulum) of liver and other tissues contain a large variety of enzymes, together called microsomal enzymes
• microsomes are minute spherical vesicles derived from endoplasmic reticulum after disruption of cells by centrifugation, enzymes present in microsomes are called microsomal enzymes.
• They catalyse glucuronide conjugation, most oxidative reactions, and some reductive and hydrolytic reactions.
• The monooxygenases, glucronyl transferase etc are important microsomal enzymes.
• Non-microsomal enzymes: Enzymes occurring in organelles/sites other than endoplasmic reticulum (microsomes) are called non-microsomal enzymes.
• These are usually present in the cytoplasm, mitochondria, etc. and occur mainly in the liver, Gl tract, plasma and other tissues.
• They are usually non-specific enzymes that catalyse few oxidative reactions, a number of reductive and hydrolytic reactions, and all conjugative reactions other than glucuronidation.
• None of the non-microsomal enzymes involved in drug biotransformation is known to be inducible.
Drug Metabolism
Factors Affecting Drug Metabolism
1. Species differences: eg in phenyl butazone, procaine and barbiturates.
2. Genetic differences – variation exist.
3. Age of animal – feeble in fetus, aged, newborn.
4. Sex: under the influence of sex hormones.
5. Nutrition: starvation and malnutrition
6. Pathological conditions: Liver/Kidney dysfunction
Phase 1 reaction. (Non synthetic phase)
• Change in drug molecule generally results in the introduction of a functional group into molecules or the exposure of new functional groups of molecules.
• Phase I (non-synthetic or non-conjugative phase) includes reactions which catalyse oxidation, reduction and hydrolysis of drugs.
• In phase I reactions, small polar functional groups like -OH, -NH2. -SH, -COOH, etc. are either added or unmasked (if already present) on the lipid soluble drugs so that the resulting products may undergo phase II reactions.
• It results in activation, change or inactivation of drug.
PHASE 1 REACTION
Phase I metabolism is sometimes called a “functionalization reaction,”
Results in the introduction of new hydrophilic functional groups to compounds.
Function: introduction (or unveiling) of functional group(s) such as –OH, –NH2, –SH, –COOH into the compounds.
Reaction types: oxidation, reduction, and hydrolysis
Enzymes:
Oxygenases and oxidases: Cytochrome P450 (P450 or CYP), Flavin containing monooxygenase (FMO), peroxidase, monoamine oxidase (MAO), alcohol dehydrogenase, aldehyde dehydrogenase, and xanthine oxidase.
Reductase: Aldo-keto reductase and quinone reductase.
Hydrolytic enzymes: esterase, amidase, aldehyde oxidase and alkyl hydrazine oxidase.
Enzymes that scavenge reduced oxygen: Superoxide dismutases, catalase,glutathione peroxidase, epoxide hydrolase, y-glutamyl transferase, dipeptidase, and cysteine conjugate β-lyase
Oxidation
Oxidation by cytochrome P450 iso enzymes (microsomal mixed-function oxidases). Oxidation by enzymes other than cytochrome P450s.
Most of these are:
(a) Oxidation of alcohol by alcohol dehydrogenase
(b) Oxidation of aldehyde by aldehyde dehydrogenase
(c) N-dealkylation by monoamine oxidase
• Oxidative reactions are most important metabolic reactions, as energy in animals is derived by oxidative combustion of organic molecules containing carbon and hydrogen atoms.
• The oxidative reactions are important for drugs because they increase hydrophilicity of drugs by introducing polar functional groups such as -OH.
• Oxidation of drugs is non-specifically catalysed by a number of enzymes located primarily in the microsomes. Some of the oxidation reactions are also catalysed by non- microsomal enzymes (e.g., aldehyde dehydrogenase, xanthine oxidase and monoamine oxidase).
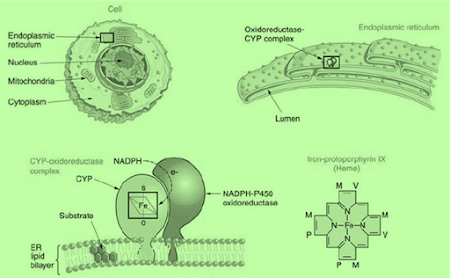
• The most important group of oxidative enzymes are microsomal monooxygenases or mixed function oxidases (MFO).
• These enzymes are located mainly in the hepatic endoplasmic reticulum and require both molecular oxygen (O2) and reducing NADPH to effect the chemical reaction.
• Mixed function oxidase name was proposed in order to characterise the mixed function of the oxygen molecule, which is essentially required by a number of enzymes located in the microsomes.
• The term monooxygenases for the enzymes was proposed as they incorporate only one atom of molecular oxygen into the organic substrate with concomitant reduction of the second oxygen atom to water.
• The overall stoichiometry of the reaction involving the substrate RH which yields the product ROH, is given by the following reaction:
MFO
RH+O2+NADPH+H+ —————-► ROH+H2O+NADP+
• The most important component of mixed function oxidases is the cytochrome P-450 because it binds to the substrate and activates oxygen.
• The wide distribution of cytochrome P-450 containing MFOs in varying organs makes it the most important group of enzymes involved in the biotransformation of drugs.
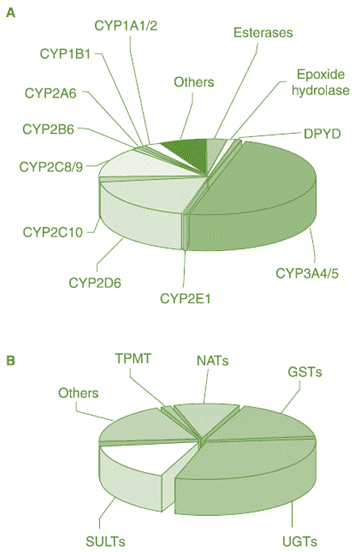
PHASE I ENZYMES
Cytochrome P450 Monooxygenase (Cytochrome P450, P450, or CYP)
• Flavin-Containing Monooxygenase (FMO)
• Esterase
• Alcohol Dehydrogenase (ADH)
• Aldehyde Dehydrogenase (ALDH)
• Monoamine Oxidase (MAO))
PHASE II ENZYMES
• Uridine Diphosphate- Glucuronosyltransferase (UDPGT)
• Sulfotransferase (ST)
• N-Acetyltransferase (NAT)
• Glutathione S-Transferase (GST)
• Methyl Transferase
• Amino Acid Conjugation
The cytochrome P-450 ENZYMES
• Super family of haem-thiolate proteins that are widely distributed across all living creatures.
• The name given to this group of proteins because their reduced form binds with carbon monoxide to form a complex, which has maximum absorbance at 450 nm.
• Depending upon the extent of amino acid sequence homology, the cytochrome P- 450 (CYP) enzymes superfamily contains a number of isoenzymes, the relative amount of which differs among species and among individuals of the same species.
• These isoenzymes are grouped into various families designated by Arabic numbers 1, 2, 3 (sequence that are greater than 40%
identical belong to the same family), each having several subfamilies designated by Capital letter A, B, C, while individual isoenzymes are again allotted Arabic numbers 1.2,3 (e.g., CYP1A1, CYP1A2, etc.).
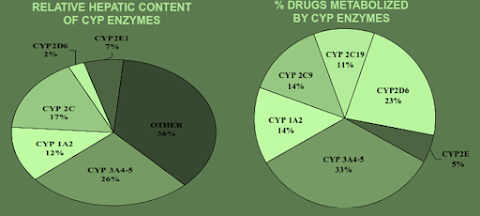
ROLE OF CYP ENZYMES IN HEPATIC DRUG METABOLISM
• In human beings, of the 1000 currently known cytochrome P-450s, about 50 are functionally active. These are categorised into 17 families, out of which the isoenzymes CYP3A4 and CYP2D6 carry out biotransformation of largest number of drugs.
Participation of the CYP Enzymes in Metabolism of Some Clinically Important Drugs
CYP | Examples |
1A1 | Caffeine, Testosterone, R-Warfarin |
1A2 | Acetaminophen, Caffeine, Phenacetin, R-Warfarin |
2A6 | 17 β -Estradiol, Testosterone |
2B6 | Cyclophosphamide, Erythromycin, Testosterone |
2C-family | Acetaminophen, Tolbutamide (2C9); Hexobarbital, S-Warfarin (2C9,19); |
2E1 | Acetaminophen, Caffeine, Chlorzoxazone, Halothane |
2D6 | Acetaminophen, Codeine, Debrisoquine |
3A4 | Acetaminophen, Caffeine, Carbamazepine, Codeine, Cortisol, |
2. Reduction
Enzymes responsible for reduction of xenobiotics require NADPH as a cofactor. Substrates for reductive reactions include azo- or nitro compounds, epoxides, heterocyclic compounds, and halogenated hydrocarbons:
(a) Azo or nitro reduction by cytochrome P450;
(b) Carbonyl (aldehyde or ketone) reduction by aldehyde reductase, aldose reductase, carbonyl reductase, quinone reductase
(c) Other reductions including disulfide reduction, sulfoxide reduction, and reductive dehalogenation.
• The acceptance of one or more electron(s) or their equivalent from another substrate.
• Reductive reactions, which usually involve addition of hydrogen to the drug molecule, occur less frequently than the oxidative
reactions.
• Biotransformation by reduction is also capable of generating polar functional groups such as hydroxy and amino groups, which can undergo further biotransformation.
• Many reductive reactions are exact opposite of the oxidative reactions (reversible reactions) catalysed cither by the same enzyme (true reversible reaction) or by different enzymes (apparent reversible reactions).
• Such reversible reactions usually lead to conversion of inactive metabolite into active
3. Hydrolysis:
Esters, amides, hydrazides, and carbamates can be hydrolyzed by various enzymes.
• The hydrolytic reactions, contrary to oxidative or reductive reactions, do not involve change in the state of oxidation of the substrate, but involve the cleavage of drug molecule by taking up a molecule of water.
• The hydrolytic enzymes that metabolise drugs are the ones that act on endogenous substances, and their activity is not confined to liver as they are found in many other organs like kidneys, intestine, plasma, etc.
• A number of drugs with ester, ether, amide and hydrazide linkages undergo hydrolysis. Important examples are cholinesters, procaine, procainamide, and pethidine.
Phase II reaction or Conjugation Reaction (Synthetic
phase)
• Last step in detoxification reactions and almost always results in loss of biological activity of a compound.
• May be preceded by one or more of phase one reaction.
• It involves conjugation of functional groups of molecules with hydrophilic endogenous substrates such as carbohydrates and amino acids (formation of conjugates) with drug or its metabolites formed in phase 1 reaction.
• Involve attachment of small polar endogenous molecules like glucuronic acid, sulphate group, methyl group, amino acids etc., to either unchanged drugs or phase I products.
• Products called as ‘conjugates’ are water-soluble metabolites, which are readily excreted from the body.
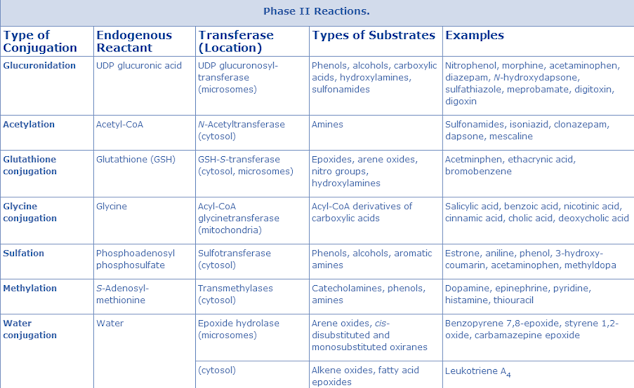
Phase II metabolism (conjugation reactions)
• Generally, the conjugation reaction with endogenous substrates occurs on the metabolite(s) of the parent compound after phase I metabolism; however, in some cases, the parent compound itself can be subject to phase II metabolism.
Function: conjugation (or derivatization) of functional groups of a compound or its metabolite(s) with endogenous substrates.
Reaction types: glucuronidation, sulfation, glutathione-conjugation, N-acetylation, methylation and conjugation with amino acids (e.g., glycine, taurine, glutamic acid).
Enzymes:
• Glucuronidation by uridine diphosphate-glucuronosyltransferase
• Sulfation by sulfotransferase
• Acetylation by N-acetyltransferase
• Glutathione conjugation by glutathione S-transferase
• Methylation by methyl transferase
• Amino acid conjugation
• Phase II or conjugation (Latin, conjugatus = yoked together) reactions involve combination of the drug or its phase I metabolite with an endogenous substance to form a highly polar product, which can be efficiently excreted from the body.
• In the biotransformation of drugs, such products or metabolites have two parts:
• Exocon, the portion derived from exogenous compound or xenobiotic,
• Endocon, the portion derived from endogenous substance.
• Conjugation reactions have high energy requirement and they often utilise suitable enzymes for the reactions.
• The endogenous substances (endocons) for conjugation reactions are derived mainly from carbohydrates or amino acids and usually possess large molecular size.
• They are strongly polar or ionic in nature in order to render the substrate water- soluble. The molecular weight of the conjugate (metabolite) is important for determining its route of excretion.
• High molecular weight conjugates are excreted predominantly in bile (e.g., glutathione exclusively, glucuronide mainly)
• While low molecular weight conjugates are excreted mainly in the urine.
• As the availability of endogenous conjugating substance is limited, saturation of this process is possible and the unconjugated drug/metabolite may precipitate toxicity.
1. Conjugation with glucuronic acid/ Glucuronidation
• Conjugation with glucuronic acid (glucuronide conjugation or glucuronidation) is the most common and most important phase II reaction in vertebrates, except cats and fish.
• The biochemical donor (cofactor) of glucuronic acid is uridine diphosphate-D- glucuronic acid (UDPGA) and the reaction is carried out by enzyme uridine diphosphate-glucuronyl transferase (UDP-glucuronyl transferase; glucuronyl transferase).
• Glucuronyl transferase is present in microsomes of most tissues but liver is the most active site of glucuronide synthesis.
• Glucuronidation can take place in most body tissues because the glucuronic acid donor UDPGA is present in abundant quantity in body, unlike donors involved in other phase II reactions.
• In cats, there is reduced glucuronyl transferase activity, while in fish there is deficiency of endogenous glucuronic acid donor.
• The limited capacity of this metabolic pathway in cats may increase the duration of action, pharmacological response and potential of toxicity of several lipid-soluble drugs (e.g., aspirin) in this species.
• A large number of drugs undergo glucuronidation including morphine, paracetamol and desipramine. Certain endogenous substances such as steroids, bilirubin, catechols and thyroxine also form glucuronides.
• Deconjugation process: Occasionally some glucuronide conjugates that are excreted in bile undergo deconjugation process in the intestine mainly mediated by β glucuronidase enzyme.
• This releases free and active drug in the intestine, which may be reabsorbed and undergo entero-hepatic cycling.
• Deconjugation is an important process because it often prolongs the pharmacological effects of drugs and/or produces toxic effects.
2. Conjugation with sulphate/ Sulphation:
• Conjugation with sulphate (sulphate conjugation, sulphoconjugation or sulphation) is similar to glucuronidation but is catalysed
by non-microsomal enzymes and occurs less commonly.
• The endogenous donor of the sulphate group is 3′-phosphoadenosine-5′- phosphosulphate (PAPS), and enzyme catalysing the reaction is sulphotransferase
• The conjugates of sulphate are referred to as sulphate ester conjugates or ethereal sulphates. Unlike glucuronide conjugation, sulphoconjugation in mammals is less important because the PAPS donor that transfers sulphate to the substrate is easily depleted.
• Capacity for sulphate conjugation is limited in pigs. However in cats, where glucuronidation is deficient, sulphate conjugation is important. Functional groups capable of forming sulphate conjugates include phenols, alcohols, aryl amines, N- hydroxyl amines and N-hydroxyamides.
• Drugs undergoing sulphate conjugation include chloramphenicol, phenols and steroids.
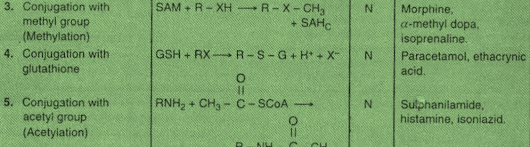
3. Conjugation with methyl group/ Methylation:
• Conjugation with methyl group (methyl conjugation or methylation) involves transfer of a methyl group (-CH3) from the cofactor S-adenosyl methionine (SAM) to the acceptor substrate by various methyl transferase enzymes.
• Methylation reaction is of lesser importance for drugs, but is more important for biosynthesis (e.g., adrenaline, melatonin) and inactivation (e.g., histamine) of endogenous amines.
• Occasionally, the metabolites formed are not polar or water-soluble and may possess equal or greater activity than the parent compound (e.g., adrenaline synthesised from noradrenaline).
4. Conjugation with glutathione and mercapturic acid formation.
• Conjugation with glutathione (glutathione conjugation) and mercapturic acid formation is a minor but important metabolic pathway in animals.
• Glutathione (GSH, G=glutathione and SH = active-SH group) is a tripeptide having glutamic acid, cysteine and glycine.
• It has a strong nucleophilic character due to the presence of a -SH (thiol) group in its structure. Thus, it conjugates with electrophilic substrates, a number of which are potentially toxic compounds, and protects the tissues from their adverse effects.
• The interaction between the substrate and the GSH is catalysed by enzyme glutathione-S-transferase, which is located in the soluble fraction of liver homogenates.
• The glutathione conjugate either due to its high molecular weight is excreted as such in the bile or is further metabolised to form mercapturic acid conjugate that is excreted in the urine.
5. Conjugation with acetyl group/ Acetylation:
• Conjugation with acetyl group (acetylation) is an important metabolic pathway for drugs containing the amino groups.
• The cofactor for these reactions is acetyl coenzyme A and the enzymes are non- microsomal N-acetyl transferases, located in the soluble fraction of cells of various tissues.
• Acetylation is not a true detoxification process because occasionally it results in decrease in water solubility of an amine and. Thus, increase in its toxicity (e.g., sulphonamides).
• Acetylation is the primary route of biotransformation of sulphonamide compounds. Dogs and foxes do not acetylate the aromatic amino groups due to deficiency of arylamine acetyl transferase enzyme.
6. Conjugation with amino acids
• Conjugation with amino acids occurs to a limited extent in animals because of limited availability of amino acids. The most important reaction involves conjugation with glycine.
• Conjugation with other amino acids like glutamine in man and ornithine in birds is also seen.
• Examples of drugs forming glycine or glutamine conjugates are salicylic acid, nicotinic acid and cholic acid.
Conjugation with thiosulphate: Conjugation with thiosulphate is an important reaction in the detoxification of cyanide. Conjugation of cyanide ion involves transfer of sulphur atom from the thiosulphate to the cyanide ion in presence of enzyme rhodanse to form inactive thiocyanate.
Thiocyanate formed is much less toxic than the cyanide (true detoxification) and it is excreted in urine.
SUMMARY
• Metabolism is an essential pharmacokinetic process, which renders lipid soluble and non-polar compounds to water soluble and polar compounds so that they are excreted by various processes.
• The major site of drug metabolism is the liver.
• Within a given cell, most drug metabolising activity is found in the smooth endoplasmic reticulum and the cytosol.
• Drug metabolism can also occur in mitochondria, nuclear envelope and plasma membrane.
• Enzymes occurring in organelles/sites other than endoplasmic reticulum (microsomes) are called non-microsomal enzymes.
• Phase I (non-synthetic or non-conjugative phase) includes reactions which catalyse oxidation, reduction and hydrolysis of drugs.
• In phase I reactions, small polar functional groups like-OH, -NH2. -SH, -COOH, etc. are either added or unmasked (if already present) on the lipid soluble drugs so that the resulting products may undergo phase II reactions.
• The name given to this group of proteins because their reduced form binds with carbon monoxide to form a complex, which has maximum absorbance at 450 nm.
• Depending upon the extent of amino acid sequence homology, the cytochrome P-450 (CYP) enzymes superfamily contains a number of isoenzymes, the relative amount of which differs among species and among individuals of the same species.
• Phase II or conjugation (Latin, conjugatus = yoked together) reactions involve combination of the drug or its phase I metabolite with an endogenous substance to form a highly polar product, which can be efficiently excreted from the body.
• Conjugation with glucuronic a./ Glucuronidation
• Conjugation with sulphate/ Sulphation
• Conjugation with methyl group/ Methylation
• Conjugation with glutathione and mercapturic acid formation
• Conjugation with acetyl group/ Acetylation
• Conjugation with aminoacids
• Conjugation with thiosulphate
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf