General Anaesthetics

Objective
At the end of this lecture, the student will be able to:
• Definition of anaesthesia
• Chart the history of general anaesthesia
• Enlist the ideal characteristics of general anaesthesia
• Describe the mode of action of general anaesthesia
• Categorize the stages of general anaesthesia
• Classify the GA according to their chemical structure
• Define the general anaesthisa
• Outline the scheme of synthesis of some GAs
• Recognize the specific uses of the various GAs
What is Anaesthesia?
• The absolute loss of sensation is termed as anaesthesia
• It is derived from a Greek word meaning insensitivity or lack of feeling
• It is a reversible condition of comfort and a state of quietness for a patient within the physiological limit before, during and after performance of a procedure
General Anaesthesia
• For surgical procedure to render the patient unaware/unresponsive to the painful stimuli.
• – Drugs producing General Anaesthesia – are called General Anaesthetics
Local Anaesthesia
• Reversible inhibition of impulse generation and propagation in nerves.
• In sensory nerves, such an effect is desired when painful procedures must be performed, e.g., surgical or dental operations
• – Drugs producing Local Anaesthesia – are called Local Anaesthetics e.g. Procaine,
History of Anaesthesia
• Pre-1846 – the foundations of anaesthesia
• 1846 – 1900 – establishment of anaesthesia
• 20th Century – consolidation and growth
• 21st Century – the future
Drug methods
• Alcohol
• Opium (poppy)
• Hyoscine (Mandrake)
• Cannabis (Hemp)
• Cocaine (New World)
Non-drug methods
• Cold
• Concussion
• Carotid compression
• Nerve compression
• Hypnosis
• Blood letting
• Middle ages the anesthetic effects of cold water and ice were recognized.
• In 17th century, Marco Aurelio Severino described the technique of “refrigeration anesthesia” in which snow was placed in parallel lines across the incisional plane such that the surgical site became insensate within minutes.
• The technique never became widely used, likely because of the challenge of maintaining stores of snow year-round.
• Manipulation of the psyche to relieve surgical pain was undertaken by French physicians Charles Dupotet and Jules Cloquet in the late 1820s with hypnosis, then called mesmerism.
• Greek physician from the first century AD, commented on the analgesia of mandragora, a drug prepared from the bark and leaves of the mandrake plant.
• He observed that the plant substance could be boiled in wine, strained, and used “in the case of persons … about to be cut or
cauterized when they wish to produce anesthesia.
•”Mandragora was still being used to benefit patients as late as the 17th century
• Alcohol was another element of the pre-ether armamentarium because it was thought to induce stupor and blunt the impact of pain
• Laudanum was an alcohol-based solution of opium first compounded by Paracelsus in the 16th century
• It was wildly popular in the Victorian and Romantic periods, and prescribed for a wide variety of ailments from the common cold to tuberculosis. it was frequently misused and abused
• Laudanum was given by nursemaids to quiet wailing infants and abused by many upper-class women, poets, and artists who fell victim to its addictive potential
• In 1773 Nitrous oxide was first prepared by Joseph Priestley
• In 1799 Davy commented that nitrous oxide transiently relieved a severe headache, obliterated a minor headache, and briefly quenched an aggravating toothache. Quoted; “As nitrous oxide in its extensive operation appears capable of destroying physical pain, it may probably be used with advantage during surgical operations in which no great effusion of blood takes place.”
• Davy’s lasting nitrous oxide legacy was coining the phrase “laughing gas” to describe its unique property
• 1818: Michael Faraday (1791-1867) described “narcotic effects” of ether 1821:
• Benjamin Brodie (1783-1862) demonstrated to Royal College of Surgeons that ether inhalation could induce insensibility in a guinea pig – “….ether acted like a narcotic poison……”
• In 1831 David Waldie suggested chloroform, which had first been prepared
• Horace Wells, a dentist, was the first person who discovered the usage of nitrous oxide as an effective surgical anaesthetic in 1844
• 1846, William Morton, a dentist, demonstrated the anaesthetic action of diethyl ether which is not commonly used now a days due to its adverse properties
• Pierre Cyprien Ore: introduced chloral hydrate in 1872
• Barbiturates were synthesised in 1603 for induction of anaesthesia
• Oct 16 1846 Gilbert Abbott underwent surgical excision of a neck tumor at the Massachusetts General Hospital in the operating room now known as “the ether dome.” The era of modern anesthesia and a revolution in the medical care of the surgical patient had begun
Chlorofom Popularised by James.Y.Simpson & practiced by John Snow
John Snow used chlorofom to deliver the last two children of Queen Victoria
• In 1934 the anesthetic properties of cyclopropane were discovered accidentally by Ralph Waters’s chemists analyzing impurities in propylene
• In 1956 came the introduction of halothane by Charles Suckling, a nonflammable volatile halogenated alkane that quickly became the dominant anesthetic
• The first intravenous anaesthetic, thiopentone, was introduced in 1935
General Anaesthetics
The Cardinal Features of General Anaesthetics
• Loss of all sensation, especially pain
• Sleep (unconsciousness) and amnesia
• Abolition of somatic and autonomic reflexes
• Immobility and muscle relaxation
• General anaesthetics bring about descending depression of the central nervous system; starting with the cerebral cortex, the basal ganglia, the cerebellum and finally the spinal cord
• 1st – Cerebral cortex
• 2nd – Basal ganglia
• 3rd – cerebellum
• 4th – spinal cord
Stage I: Stage of Analgesia or cortical stage
• Mild depression of higher cortical centers occurs
• Starts from beginning of anaesthetic inhalation and lasts upto the loss of consciousness
• Pain is progressively abolished during this stage
• Patient remains conscious, can hear and see, and feels a dream like state
• Reflexes and respiration remain normal
• It is difficult to maintain -use is limited to short procedures only
Stage II: Stage of Delirium and Excitement
• From loss of consciousness to beginning of automatic breathing or entry into surgical anesthesia stage
• This is accompanied by excitement, delirium, uncoordinated muscular movement and breath holding
• Like: Eyelash reflex disappear
• Excitement -patient may shout, struggle and hold his breath
• Muscle tone increases, jaws are tightly closed
• Breathing is jerky; vomiting, involuntary micturitionor defecation
Stage III: Stage of Surgical anaesthesia
• Extends from onset of spontaneous respiration to respiratory paralysis, that movement ceases
• This has been divided into 4 planes:
– Plane 1: Roving eye balls. This plane ends when eyes become fixed
– Plane 2: Loss of corneal and laryngeal reflexes
– Plane 3: Pupil starts dilating and light reflex is lost. This was the desired phase of surgery when muscle relaxant were not used
– Plane 4: Intercostalparalysis, shallow abdominal respiration, dilated pupil
The four planes described in other words
Plane-1) spinal reflexes are lost
Plane-2) muscle reflexes are lost
Plane-3) paralysis of intercostal muscles occur
Plane-4) muscle tone disappears
Stage IV: Medullary/respiratory paralysis
• Cessation of breathing -failure of circulation -death
• Pupils: widely dilated
• Muscles are totally flabby
• Pulse is imperceptible
• BP is very low
• This stage is the toxic or overdose stage- Death occurs
• No stimulus or operative procedure carried out during this stage.
• Potentially dangerous responses can occur during this stage including vomiting, laryngospasm and uncontrolled movement.
• This stage is not found with modern anaesthesia– preanaesthetic medication, rapid induction etc
Ideal characteristics of General Anaesthetics:
• Rapid and pleasant induction and withdrawal of surgical anesthesia
• Adequate relaxation of skeletal muscle
• Safe, non-toxic, free from adverse effects
• Should me noninflammable, non-explosive
• Compatible with anesthetic devices – Inert
• Inexpensive
• Non-irritating to mucous membrane
• Should not produce hypotension, nausea and vomiting
• Compatible with adjuvant drugs used in anesthesia
Classification of General Anaesthetics:
Inhalation Anaesthetics:
• They could be either volatile liquids or gases and they are administered through inhalation process
• They may be halogenated, non-halogenated compounds or gaseous in nature
• Halogenated: I. Hydrocarbons e.g. : Halothane, Chloroform
II. Ethers: e.g.: Methoxy flurane, Enflurane, Isoflurane
• Non halogenated: I. Hydrocarbon e.g.: Cyclopropane
II. Ethers e.g.:
Diethyl ether, vinyl ether
• Gaseous e.g.: Nitrous oxide
Injectable Anaesthetics or nonvolatile anaesthetics: Which are administered in the form of injection through intravenous route
• Ultra short acting barbiturates: e.g.: Methohexital sodium, Thiopental sodium
• Dissociative Anaesthetics: e.g.: Ketamine hydrochloride
Halothane INN, BAN, USAN
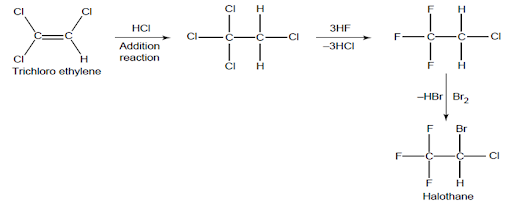
• Chemical name: 2bromo-2chloro-1,1,1trifluoroethane; Ethane, 2 bromo2-chloro-1,1,1-trifluoro:
• Official In: BP, U.S.P, Eur.P
• Marketed as Fluothane (Ayerst) and Halothane (May and Baker)
• It is the only inhalational anesthetic containing bromine
Chemical Structure of Halothane
Properties:
• It is a clear, colourless, heavy, non-flammable liquid, slightly soluble in water, miscible with ethanol, and with trichloroethylene
Medicinal Uses
• It is a relatively safe potent volatile anaesthetic administered by inhalation
• It is twice as potent as chloroform and 4 times that of ether
• It may produce any depth of anaesthesia without causing hypoxia
• It reduces the blood pressure and frequently decreases the pulse rate and depresses respiration.
• It induces muscle relaxation and reduces pains sensitivity by altering tissue excitability
Mechanism of action of Halothane:
• Halothane causes general anaethesia due to its actions on multiple ion channels, which ultimately depresses nerve conduction, breathing, cardiac contractility
• Its immobilizing effects have been attributed to its binding to potassium channels in cholinergic neurons
• Halothane’s effect are also likely due to binding to NMDA and calcium channels, causing hyperpolarization
Halothane synthesis
• Route 1
• Route 2
Particular aspects of the use of Halothane:
• Moderate muscular relaxation is produced, but is rarely sufficient for major abdominal surgery
• It potentiates the action of neuromuscular blockers
• Heat loss is accelerated
• It is useful in bronchitic and asthmatic patients.
• It is a potent, relatively safe general inhalation anaesthetic used in conjunction with N2O
• For skeletal muscle relaxation, it is used with succinyl choline or tubocurarine
Advantages of Halothane:
• It is a volatile liquid halogenated hydrocarbon
• It is rapidly acceptable drug because of its non-inflammable nature
• It has high potency and relatively lower blood/gas partition coefficient
Disadvantages of Halothane:
• It has narrow margin of safety
• It has high incidence of hepatic necrosis hence reduced in its use
Storage: It should be stored in well-closed airtight containers, protected from light, at a temperature not exceeding 25°C in a nonreactive metal container
Methoxyflurane: INN, BAN, USAN,
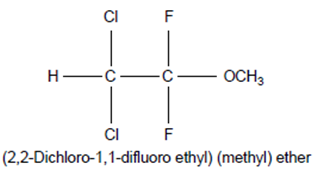
• Chemical Formula: CHCl2CF2—O—CH3
• Chemical Name: 2, 2-Dichloro-1, 1-difluoroethyl methyl ether; Ethane, 2, 2-dichloro-1, 1, 1-difluoro-1-methoxy-;
• Official In: B.P., B.P.C., U.S.P., N.F.
Chemical Structure of Methoxyflurane
Properties:
• It is a clear, colourless liquid, noninflammable and nonexplosive in air or oxygen in anaesthetic concentrations.
Medicinal Uses
• It is one of the most potent anaesthetic agents frequently used in practice today
• In fact, it is employed to cause comparatively light anaesthesia with deep analgesic and muscle relaxation, features which make it convenient for short surgical operations, e.g., obstetrics
Mechanism of action of Methoxyflurane:
• Methoxyflurane induces a reduction in junctional conductance by decreasing gap junction channel opening times and increasing gap junction channel closing times
• Methoxyflurane also activates calcium dependent ATPase in the sarcoplasmic reticulum by increasing the fluidity of the lipid membrane
• It also appears to bind the D subunit of ATP synthase and NADH dehydogenase
• Methoxyflurane also binds to the GABA receptor, the large conductance Ca2+ activated potassium channel, the glutamate receptor and the glycine receptor
Enflurane
• Chemical Formula: CFClH-CF2-O-CF2H
• Chemical Name: 2-chloro-1,1,2,-trifluoroethyl-difluoromethyl ether
• Official: USP
• Developed by Ross Terrell in 1963, It was first used clinically in 1966.
• It was increasingly used for inhalational anesthesia during the 1970s and 1980s but is no longer in common use due to less potency
Chemical Structure of Enflurane
Properties:
• Enflurane is a structural isomer of isoflurane
• It is a clear, colourless, volatile liquid with pleasant hydrocarbon-like odour
• Soluble in water, miscible with organic solvents, chemically it is extremely stable.
• The induction of an emergence from anaesthesia and adjustment of anaesthetic depth during maintenance is smooth and moderately rapid.
• It is a noninflammable halogenated ether anaesthetic and provides rapid induction with no excitement
Isoflurane
Chemical Formula: C3H2ClF5O
Chemical Name: 1chloro-2,2,2trifluoro-difluoro-methylether
Official: USP
Chemical Structure of Isoflurane
Properties:
• It is available as clear, colourless liquid at room temperature, with sweet taste
• It is miscible with organic liquids including fats and oils. Isoflurane is non-flammable and nonexplosive
Medicinal Uses
• General inhalation anesthetic drug
Desflurane:
• Chemical Name: 1,2,2,2-tetrafluoroethyl difluoromethyl ether
• Chemical Formula: C3H2F6O
Chemical Structure of Desflurane
• It is a highly fluorinated methyl ethyl ether used for maintenance of general anesthesia. Like halothane, enflurane, and isoflurane, it is a racemic mixture of (R) and (S) optical isomers (enantiomers).
Properties:
• Desflurane is a colorless, volatile liquid below 22.8°C
• It has a lower solubility in rubber and plastic than halothane, isoflurane, or sevoflurane
• Desflurane is quite irritating to the airway and therefore it is not suitable for an inhalation induction.
Sevoflurane:
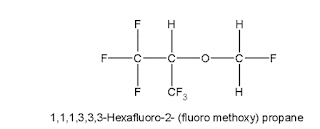
Chemical Name: 1,1,1,3,3,3,hexafluro-2- fluro methoxy propane
Chemical Formula: C4H3F7O
Chemical Structure of Sevoflurane
Properties:
• Low boiling liquid with a slight odour; miscible with most organic solvents including fats or oils; practically insoluble in water. It is a non-flammable, nonirritating agent
Medicinal Uses:
Sevoflurane is a non-explosive inhalation anesthetic used in the induction and maintenance of general anesthesia
II Nonvolatile or intravenous anaesthetics
• Intravenous anaesthetics usually cause unconsciousness when administered parenterally.
• However, the duration of action can be safely monitored depending on the amount of drug administered.
• Produced rapid onset of action within seconds and the duration of action is for 30 sec.
• The rapid onset of action is attributed due to rapid partition from blood across blood brain barrier to CNS
Ultra short acting barbitutrates:
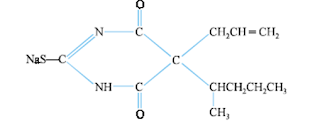
Methohexital Sodium USAN, Methohexitone Sodium BAN,
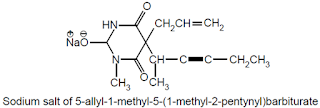
• Chemical Name: Sodium 5 allyl-1-methyl-5-(1-methyl-2-pentynyl) barbiturate; 2, 4, 6 (1H, 3H, 5H) – Pyrimidinetrione, 1-methyl-5-(1- methyl-2-pentynyl)-5-(2-propenyl)-, Sodium salt, USP
• It has extensive hydrophobic character and lipid/water partition coefficient so compound penetrates into CNS in few second after IV injection and redistributed to other body site
• It undergoes rapid metabolic inactivation.
– Racemic mixture is used.
Chemical structure of methohexital sodium
Dose
• For intravenous and rectal route 10mg/ml is used.
• For continuous intravenous administration 2mg/ml is used.
• For intramuscular 50mg/ml is used.
Properties: White to off-white hygroscopic powder, essentially odourless, and the solution is alkaline to litmus, soluble in water.
• Methohexital produces more rapid recovery from unconsciousness than thiopental.
Uses: It is more potent and has shorter duration of action.
• It is used for the induction of anaesthesia through the intravenous administration.
Advantages: It has two advantages over thiopental sodium.
• First, being it has less affinity towards fatty tissues and second, it has a greater potency.
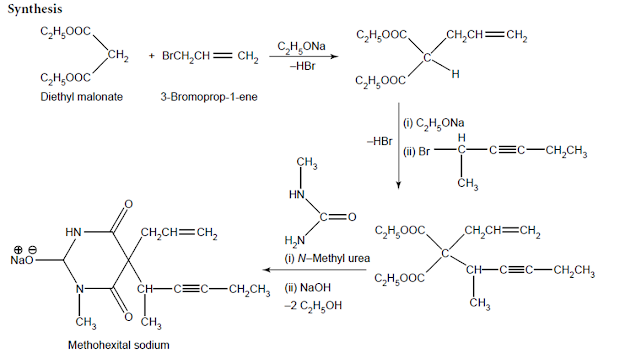
Synthesis of Methohexital sodium:
• Grignardization of 1-butynyl magnesium bromide with acetaldehyde and subsequent treatment of the resulting alcohol with PCl5 yields 2-chloro-3-pentyne. Now, ethyl (1-methyl-2- pentynyl) – cyanoacetate is obtained
• Therefrom by its condensation with ethyl cyanoacetate in the presence of sodium ethylate.
• Further condensation of the resulting product with allyl bromide gives rise to ethyl-(1-methyl- 2-pentynyl) allylcyanoacetate.
• Condensation with N-methyl urea and subsequent neutralization with NaOH produces methohexital sodium.
Medicinal uses
• Its onset of action is quite speedy comparable to thiopental sodium while its recovery is more rapid.
• For these reasons, this intravenous anaesthetic is specifically useful for short surgical operations, such as oral surgery, gynaecological investigation, genitourinary procedures, and electroconvulsive therapy.
Thiopental sodium
Thiopental sodium belonging to the class of ultrashort-acting barbiturates (e.g., thiopental)
• are mostly employed IV to cause a rapid onset of unconsciousness for both surgical and basal anaesthesia
• Importantly, it may be used first and foremost to cause anaesthesia, which subsequently should be adequately sustained as well as maintained in the course of a surgical operative procedure with the aid of a general anaesthetic
It has high lipid water partition coefficient which forms the base for the rapid partitioning.
• It can also be used in the combination with other anaesthetic agent such as nitrous oxide.
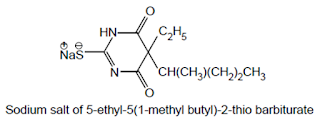
Thiamylal Sodium USAN, Sodium Thiamylal BAN:
• Chemical Name: Sodium 5-allyl-5-(1-methylbutyl)-2-thiobarbiturate; 4, 6-(1H, 5H)-Pyrimidine-dione, dihydro- 5-(1-methylbutyl)-5-(2-propenyl)-2-thioxo-, monosodium salt; U.S.P.,
• It is an ultra-short acting barbiturate mainly used for intravenous anaesthesia in conditions of comparatively short-duration.
• It is also effective for the termination of convulsions of unknown origin
Chemical structure of Thiamylal Sodium
• Thiomylal is a highly hydrophobic thiobarbiturate having its structural featuresvery much related to thiopental.
• Its biological activities are almost identical to thiopental.
• Used as intravenous anaesthetic
III. Dissociative anaesthesia
• A form of general anesthesia, but not necessarily complete unconsciousness, characterized by catalepsy, catatonia, and amnesia
– Especially produced by phenylcyclohexylamine compounds, including ketamine
• A unique anesthesia characterized by analgesia and amnesia with minimal effect on respiratory function.
• The patient does not appear to be anesthetized and can swallow and open eyes but does not process information.
• This form of anesthesia may be used to provide analgesia during brief, superficial operative procedures or diagnostic processes.
• It produces sense of dissociation from events being experienced – i.e.produces feelings of detachment from the environment and self.
• It also reduces pain and overall feeling.
• Followed by anaesthesia, analgesia and sometimes amnesia.
• Anaesthesia produced is called as cataleptic anaesthesia-
– a condition of diminished responsiveness usually characterized by a trancelike state and constantly maintained immobility
• It can used as a sole agent for surgical procedure in children.
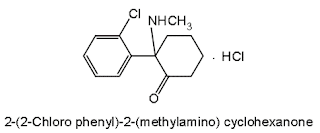
Ketamine Hydrochloride INN, BAN, USAN
• Chemical Name: 2-(o-Chlorophenyl)-2-(methylamino) cyclohexanone hydrochloride; Cyclohexanone, 2-(2- chlorophenyl)-2- (methylamino)-,hydrochloride; U.S.P., N.F
Chemical structure of Ketamine
• Properties and uses: It is a white or almost white crystalline powder, freely soluble in water, methanol, and ethanol.
• Its another name is ‘dissociative anaesthetic’ because it produces unpleasant hallucinations and strong feelings of dissociation from the environment. It is a rapidly acting nonbarbiturate general anaesthetic that produces anaesthesia and is characterized by profound analgesia
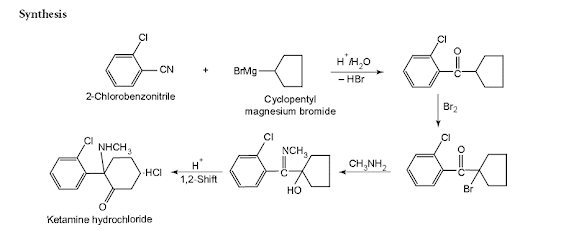
Synthesis of Ketamine hydrochloride:
• It is prepared first by the interaction of o-chlorobenzonitrile and bromo-cyclopentane in the presence of strong alkali to yield an epoxy compound.
• Secondly, the resulting epoxy compound on treatment with methylamine forms an imine
• Which undergoes molecular rearrangement upon heating in the presence of hydrochloric acid to yield ketamine hydrochloride.
• Ketalar: Phencyclidine derivative
• It is a tasteless, odorless drug that can be a powder or liquid. Racemic mixture is used
• Used for trauma patients with very unstable, low blood pressure or for elderly patients, or children.
– Emergence may be accompanied by delirium, exciteme disorientation, and confusion.
– Effect lasts for 30-60 minutes
Summary
• Agents which produce insensibility by successive or progressive depression of central nervous system
• All GAs act on the mid-brain reticular activating system and cerebral cortex to produce complete but reversible loss of consciousness
• They should be nontoxic, inert and should induce anaesthesia smoothly
• The 4 stages of anaesthesia are
– Analgesia stage
– Delirium stage
– Surgical anesthesia
– Medullary depression
• General anaesthetics are broadly classified as inhalation and IV anaesthetics
• Most of the GA also produce adequate skeletal muscle relaxation
• Dissociative anaesthetics cause dissociation from the suroundings-cataleptic anaesthesia
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf