HYDROGEN BONDING
Contents
• Introduction
• Hydrogen bonding
• Molecular size
• Rotatable bonds
• Bulk physical properties
• Lipinski Rule of Five
• The Drug Design Conundrum
Intended
learning outcomes
At the end of this lecture, student will be able to:
• Explain the importance of hydrogen bonding of drug
molecules on biological action
Hydrogen
bonding
• To form Intermolecular hydrogen bonds a hydrogen bond
between a donor and acceptor group, both the donor and the acceptor must first
break their hydrogen bonds to surrounding water molecules
• The position of this equilibrium depends on the relative
energies of the species on either side, and not just the energy of the donor-
acceptor complex
• Intramolecular hydrogen bonds are more readily formed in
water – they are entropically more favourable.
Hydrogen
bonding and bioavailability
Remember! Most oral drugs are absorbed through the gut wall
by transcellular absorption.
• De-solvation and formation of a neutral molecule is
unfavourable if the compound forms many hydrogen or ionic bonds with water.
• So, as a good rule of thumb, you don’t want too many
hydrogen bond donors or acceptors, otherwise the drug won’t get from the gut
into the blood.
• There are some exceptions to this – sugars, for example,
but these have special transport mechanisms.
Molecular
size
Molecular size is one of the most important factors
affecting biological activity, but it’s also one of the most difficult to
measure.
There are various ways of investigating the molecular size,
including measurement of:
• Molecular weight (most important)
• Electron density
• Polar surface area
• Van der Waals surface
• Molar refractivity
Molecular
weight
Number of
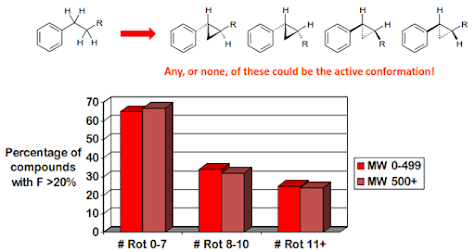
rotatable bonds
A rotatable bond is defined as any single non-ring bond,
attached to a non-terminal, non-hydrogen atom. Amide C-N bonds are not counted
because of their high barrier to rotation.
The number of rotatable bonds influences, in particular, bioavailability
and binding potency.
Remember δG = δH – TδS ! A molecule will have to adopt a
fixed conformation to bind, and to pass through a membrane. This involves a
loss in entropy, so if the molecule is more rigid to start with, less entropy
is lost. But beware!
Bulk
physical properties
When a compound is nearing nomination for entry to clinical
trials, we need to look at:
• Solubility, including in human intestinal fluid
• Hygroscopicity, i.e. how readily a compound absorbs water from
the atmosphere
• Crystalline forms – may have different properties
• Chemical stability (not a physical property! Look at
stability to pH, temperature, water, air etc)
How can these be
altered?
• Different counter ion or salt
• Different method of crystallisation
This seems
like a lot to remember!
There are various guidelines to help, the most well-known of
which is the Lipinski Rule of Five
• Molecular weight < 500
• logP < 5
• < 5 H-bond donors
• < 10 H-bond acceptors
An additional rule was proposed by Veber
• < 10 rotatable bonds
Otherwise absorption and bioavailability are likely to be
poor. This is for oral drugs only.
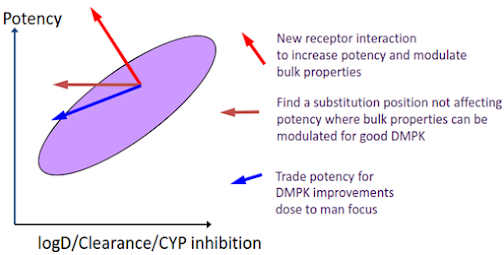
The Drug
Design Conundrum
The conundrum is that while pharmacokinetic properties
improve by modulating bulk properties, potency also depends on these –
particularly lipophilicity. There are then three approaches that could be
adopted.
SUMMARY
• As a good rule of thumb, you don’t want too many hydrogen
bond donors or acceptors, the drug won’t get from the gut into the blood.
• There are some exceptions to this – sugars, for example,
but these have special transport mechanisms.
• Relation between hydrogen bonding and bioavailability is
discussed.
• Effect of molecular weight, no of rotatable bonds, other
bulk properties on drug action is studied.
• Most oral drugs have molecular weight < 500