Regulation of Lipid metabolism

Objective
• At the end of this lecture, student will be able to
– Explain hormonal regulation of lipid metabolism
– Explain fatty acid biosynthesis and its regulation
– List the factors involved in regulation of lipid metabolism
Regulation in General
• A) Short term (response time of minutes or less):
– substrate availability
– allosteric interactions
– covalent modifications (phosphorylation/dephosphorylation)
• B) Long-term (response time of hours or days):
– changes in the rate of protein (enzyme) synthesis or breakdown
Regulation of Lipid metabolism
• Regulation – in response to the differing energy needs and dietary states of an organism
• Pancreatic a cells respond to the low blood glucose concentration of the fasting and energy-demanding states by secreting glucagon; the β cells respond to the high blood glucose concentration of the fed and resting states by secreting insulin
• Targets: enzymes of FA synthesis and FA oxidation
Lipid Metabolism
Main processes:
• Digestion, absorption, and transport of dietary fat
• Generation of metabolic energy from fat: a) lipolysis, b) β-oxidation
• Storage of excess fat in adipose tissue
Absorption and Transport
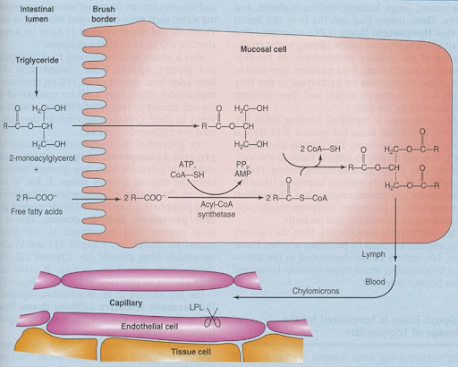
The main products of fat digestion are free FA and 2-monoacylglycerols (produced by the action of pancreatic lipase)
After absorption, FA is activated to acyl-coenzyme A (in the ER) which then reacts with 2-monoacylglycerol to form triacylglycerol
In the ER, TGs are assembled into chylomicrons that are collected by the lymph and carried to the blood stream
• TGs in chylomicrons are utilized by adipose tissue, heart, skeletal muscle, lactating mammary gland and, to a lesser extent, by the spleen, lungs, kidney.
• These tissues (but not the liver and brain) express lipoprotein lipase (LPL), attached to the surface of the capillary endothelium, that hydrolyzes TGs to FA and 2-monoacylglycerols; the products are taken up by the cells
Regulation at the Level of LPL
• In the adipose tissue, the amount of LPL is increased by feeding/ insulin and decreased by starvation
• In contrast, the amount of LPL in heart is decreased by insulin and increased by starvation
• Dietary fat is directed mainly to the adipose tissue (for storage) in the well-fed state but to the muscles during fasting (for oxidation)
FA release from Adipose Tissue
• Hormone-sensitive lipase converts the fat stored in adipose tissue into glycerol and FAs that are transported to distant sites bound to serum albumin (liver and intestine release lipids in the form of lipoproteins)
• Hydrolysis rate controls the concentration of FAs in the blood and thus regulates FA oxidation
Regulation at the Level of Hormone- Sensitive Lipase
• Norepinephrine, epinephrine, and glucagon released during physical exercise, stress, or fasting stimulate lipolysis through the β-receptors, cAMP, PKA (Protein Kinase A), and HSL(Hormone Sensitive Lipase)
Þ blood FA levels
Þ stimulation of β-oxidation in other tissues (liver, muscle)
Þ stimulation of production of ketone bodies in the liver
Mechanism
• In the resting state, the hormone-sensitive lipase is cytoplasmic and the surface of the fat droplet is covered by the protein perilipin
• The cAMP-stimulated protein kinase A phosphorylates both perilipin and lipase Þ perilipin detaches from the fat droplet, while lipase binds to the fat droplet
Insulin is released after eating and signals the abundance of dietary nutrients (Glucose, Fatty acids and Amino acids), that are eligible for storage
– Insulin inhibits HSL through phosphodiesterase degrading cAMP
Thus, the glucagon: insulin ratio is of prime importance in regulation of lipid metabolism
Glucocorticoids, growth hormone, and the thyroid hormones facilitate lipolysis by inducing the synthesis of lipolytic proteins:
β-oxidation
• FAs are activated to acyl-CoA by enzymes on the ER membrane and transported into the mitochondrion by carnitine
• β-oxidation produces:
– acetyl-CoA, NADH, FADH2
Regulation of FA Oxidation
• A) Use of FAs by the tissues is proportional to the plasma FFA level; therefore, FA oxidation is regulated at the level of HSL
– During fasting, the hormonal stimulation of adipose tissue lipolysis (HSL) provides a large amount of FA
– FA are rather oxidized (than esterified) in the liver because of an increased activity of CPT1 (see below) – acetyl-CoA formed by β-oxidation is not used for biosynthesis during fasting, its oxidation by the TCA cycle is minimal, and it is used preferentially for the synthesis of ketone bodies
• After a carbohydrate-rich meal
During fasting
• B) Carnitine-palmitoyl transferase I (CPT1) is inhibited by malonyl-CoA that is formed in the FA biosynthesis by acetyl-CoA carboxylase Þ β-oxidation is inhibited when FA synthesis is active
– Thus, in the fed state, nearly all FAs entering the liver are esterified to acylglycerols and transported out of the liver in the form of VLDL
– When FA level increases with the onset of starvation, ACC is inhibited by acyl-CoA and malonyl-CoA decreases Þ stimulation of β oxidation
FA Biosynthesis
• On a high-carbohydrate diet excess energy is stored in the form of fat
• In the liver, lactating mammary gland, and, to a lesser extent, in the adipose tissue
• FA synthesized in the liver are esterified to TGs which are released in the form of VLDL
• VLDL are utilized by the action of LPL (mainly in the adipose tissue)
• The formation of malonyl-CoA from acetyl-CoA is an irreversible process, catalyzed by acetyl-CoA carboxylase – a multifunctional polypeptide.
• It contains a biotin prosthetic group covalently bound to the amino group of a Lys residue in one of the 3 polypeptides (or domains) of the enzyme molecule.
• The two-step reaction catalyzed by this enzyme -The carboxyl group, derived from bicarbonate (HCO3), is first transferred to biotin in an ATP dependent reaction. The biotinyl group serves as a temporary carrier of CO2, transferring it to acetyl-CoA in the second step to yield malonyl-CoA.
• Mainly at the level of acetyl-CoA carboxylase (ACC):
• The long carbon chains of fatty acids are assembled in a repeating four-step sequence.
• A saturated acyl group produced by this set of reactions becomes the substrate for subsequent condensation with an activated malonyl group.
• With each passage through the cycle, the fatty acyl chain is extended by two carbons.
• When the chain length reaches 16 carbons, the product (palmitate,) leaves the cycle.
• Carbons C-16 and C-15 of the palmitate are derived from the methyl and carboxyl carbon atoms, of an acetyl-CoA used directly to prime the system at the outset; rest of the carbon atoms are derived from acetyl-CoA via malonyl-CoA
• Carbons C-16 and C-15 of the palmitate are derived from the methyl and carboxyl carbon atoms, of an acetyl-CoA used directly to prime the system at the outset; rest of the carbon atoms are derived from acetyl-CoA via malonyl-CoA
• Both the electron-carrying cofactor and the activating groups in the reductive anabolic sequence differ from those in the oxidative catabolic process –the reducing agent in the synthetic sequence is NADPH and the activating groups are two different enzyme-bound OSH groups.
• All the reactions are catalyzed by a multienzyme complex, fatty acid synthase.
The overall process of palmitate synthesis
The fatty acyl chain grows by two-carbon units donated by activated malonate, with loss of CO2 at each step.
The initial acetyl group is shaded yellow, C-1 and C-2 of malonate are shaded pink,and the carbon released as CO2 is shaded green.
After each two-carbon addition,reductions convert the growing chain to a saturated fatty acid of four, then six, then eight carbons, and so on. The final product is palmitate .
Regulation of fatty acid synthesis
1) Acetyl-CoA carboxylase is allosterically activated by citrate and inhibited by CoA-thioesters of long-chain FAs such as palmitoyl-CoA (well-fed liver has a higher citrate level and lower acyl-CoA level than does the fasting liver)
Acetyl-CoA must be converted to citrate to get from the mitochondrion into cytoplasm
• 2) acetyl-CoA carboxylase is stimulated by insulin and inhibited by glucagon and epinephrine
– glucagon and epinephrine mediate activation of the cAMP-dependent protein kinase A, which inactivates ACC
– insulin antagonizes this cascade by inducing phosphodiesterase that degrades cAMP
– insulin stimulates the synthesis of ACC and fatty acid synthase, starvation inhibits it (long-term regulation)
• 3) acetyl-CoA carboxylase is inhibited by phosphorylation by the AMP-activated protein kinase (AMPK)
– AMPK is activated when the cellular energy charge is dange–rously low (high AMP/ATP ratio) and helps the cell to survive the energy shortage by switching-off non-essential biosynthetic pathways such as FA synthesis
– In the liver, AMPK is inhibited by insulin
Regulation of ACC – Overview
Long-term Regulation
• Starvation and/or regular exercise, by decreasing the glucose concentration in the blood, change the body‘s hormone balance
• This results in long-term increases in the levels of FA oxidation enzymes (heart LPL) accompanied by long-term decreases in those of lipid biosynthesis (ACC, fatty acid synthase)
FAQs
1. What role do lipids play in the human body?
- Lipids serve as energy stores, structural components of cell membranes, and precursors to hormones and signaling molecules.
2. How does lipid metabolism differ from carbohydrate metabolism?
- While both involve energy regulation, lipid metabolism focuses on storing energy for long-term use, whereas carbohydrate metabolism provides more immediate energy.
3. Can lipid metabolism be influenced by diet?
- Yes, dietary factors such as fat intake and carbohydrate composition can affect lipid metabolism and overall metabolic health.
4. What are some common disorders associated with dysregulated lipid metabolism?
- Dyslipidemia, obesity, metabolic syndrome, and atherosclerosis are among the disorders linked to impaired lipid metabolism.
5. How can individuals maintain healthy lipid metabolism?
- Adopting a balanced diet, regular exercise, and avoiding excessive alcohol consumption can promote healthy lipid metabolism and reduce the risk of metabolic disorders.
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf