Analeptics
• The drug which increase the activity of various subunit or parts
of CNS (brain and spinal cord) are called CNS stimulant.
• in CNS there are collection of cell which perform certain definite
function are called as an center .e.g. respiratory center, vomiting center,
heat regulating center, appetite controlling center.
• Some type of drugs show mild CNS stimulant effect and produce
toxic side effect in slight higher dose e.g. strychnine.
• Other type of drug show mild CNS. stimulant effect as a side
effect. e.g. Local Anesthetics (cocaine)
• Sympathomimetics (dexamphetamine), Parasympatholytic (atropine)
• CNS stimulant mainly used as an 1) Analeptics 2) Respiratory
stimulant
• They are also useful to increase mental alertness and produce wakefulness
conditions.
• To control appetite through CNS stimulant effect.
CNS stimulants Classification
CNS
stimulants can be classified as
1)
Naturally
occurring drugs
a)
Alkaloids
:
i) Xanthine derivatives – Caffeine,
Theophylline, Theobromin
ii) Other alkaloids –Strychnine, Lobeline
2) Synthetic drugs –Nikethamide,
Picrotoxin
3) Miscellaneous drugs – Cocaine (local
Anesthetics), Dexamphetamine (Sympathomimetics), Atropine
(Anticholinergics)
• Analeptics are CNS stimulants which
are used to reduce narcosis produced by CNS depression.
• These drugs also act as respiratory
stimulants and thus are used to counteract drugs –induced Respiratory
Depression.
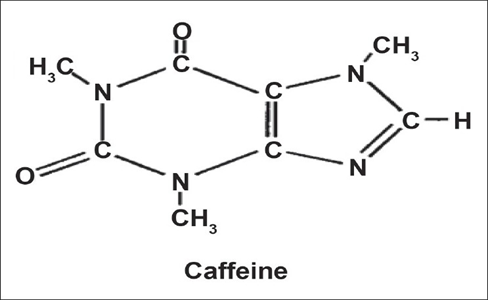
Caffeine
Structure of Caffeine:
Chemical
name of Caffeine: 1, 3, 7 trimethyl Xanthine.
Physical properties of Caffeine:
• It occurs as white crystalline powder
or silky white crystals.
• It is odorless.
• It is bitter in taste.
• When caffeine is recrystallised from
water it contains one molecule of water of crystallization.
• It is sparingly soluble in alcohol
Chemical properties of Caffeine:
• When its solutions are treated with few drops of saturated tannic
acid, solution a precipitate is produced which dissolves in excess of the
reagent.
• When few drops of iodine solution is added to its solution, a clear
solution is obtained which on acidifying with dil.HCL give brown ppt.
Stability and storage of Caffeine:
• It is decomposed by strong solution of caustic
alkalies.
• Its salts are decomposed by water. Hence it is
stored in tightly closed containers.
Uses of Caffeine:
1. It is CNS
stimulant and hence it is used
• To produce state of wakefulness.
• To enhance mental activity
• To get relief from fatigue and mild depression.
• To cause insomnia in moderate dose.
2. It has weak diuretic activity.
3. In
combination with ergotamine, it is used to get relief from migraine.
4. It
stimulate respiratory center.
Pharmaceutical formulation of Caffeine:
• Aspirin and caffeine tablets.
• Caffeine and sodium benzoate injection
• Caffeine iodide elixir
• Caffeine citrate tablets
Brand name of Caffeine:
• Anacin
• Coldarin
• Powerin
Theophylline
Structure of Theophylline:
Chemical Name of Theophylline: 1,3-dimethylxanthine or 1,3-dimethyl-7H-purine-2,6-dione
Properties of Theophylline:
• It is white crystalline powder.
• It is odorless
• It has bitter taste
• It is slightly soluble in water but
soluble in solution of alkali hydroxide
Chemical properties of Theophylline:
• When Theophylline is treated with silver
nitrate, it converted to silver Theophylline with the liberation of nitric
acid.
Stability band storage of Theophylline:
• Theophylline
is stored in well closed containers.
• Aminophylline
absorb carbon dioxide with the liberation of Theophylline and affected by light.
• Hence it
is stored in well closed light resistant containers.
Uses of Theophylline:
1.
It is potent
bronchodilator hence it is used
• To control acute and chronic asthma
• To treat chronic obstructive lung disease
• To control acute Bronchospasm
2.
It has also diuretic action
3.
It has also stimulant action by cerebrum
4.
It is cued as respiratory stimulant in
neonatal apnoea.
Pharmaceutical formulation of Theophylline:
• Aminophylline injection
• Aminophylline suppositories
• Aminophylline tablets
Brand
names of Theophylline:
• Asthmagen
• Bronkasma
• Minophylline
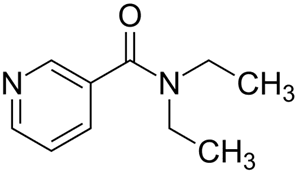
Coramine
Structure of Coramine:
Chemical names of Coramine: N, N diethyl, pyridine -3-carboxamide
Properties of Coramine:
• It occurs as a colourless or slightly yellow oily liquid or
crystalline mass.
• It has faint aromatic odour
• It has slightly bitter taste.
• It cause burning sensation and leaves faint warm sensation on
tongue
• It is miscible in water, alcohol, and ether.
Chemical properties of Coramine:
• When it is heated with sodium hydroxide solution, it decomposed to
give diethyl amine
• When it is heated strongly with sodium carbonate, it gives an
odour of pyridine.
Stability and storage of Coramine:
• It is affected by light and hence it is stored in tightly closed,
light resistant containers.
Uses of Coramine:
• It is used as an respiratory stimulant
• It is weak analeptics and hence is
used to overcome CNS depression, respiratory depression, and circulatory
failure
Pharmaceutical formulation of Coramine:
• Nikethamide injection
Brand name of Coramine:
• Coramine
• Nikethyl
Dexamphetamine
Structure of Dexamphetamine:
Chemical names of Dexamphetamine: (2S)-1-Phenylpropan-2-amine
Properties of Dexamphetamine:
• It is official as sulphate salt, which
is white crystalline powder.
• It is odourless and has saline and
slightly bitter taste.
• It is soluble in water.
• It is dextrorotatory and is 3 to 4
times more potent than levo isomers.
Stability and Storage of Dexamphetamine:
It is affected by light and hence
stored in well closed, light resistant containers
Uses of Dexamphetamine:
• It is indirectly acting
sympathomimetics with strong CNS stimulant action. it is used to treat
• Narcolepsy
• Attention deficit disorder in children
• Exogenous obesity as it has anorexiant
effect.