Carbohydrates
Definition, Classification and General Chemical Tests

Objectives
• At the end of this lecture, student will be able to:
• Define carbohydrates
• Classify carbohydrates
• Discuss the general chemical tests for carbohydrates
Carbohydrates
Definition
• (CH2O)n
• Polyhydroxy aldehydes or polyhydroxy ketones or compounds that on hydrolysis produce either of the above
Classification of Carbohydrates
• Saccharides (Crystalline, soluble in water and sweet)
– Mono saccharides and oligosaccharides
• Ploysaccharides (Amorphous, tasteless, less soluble in water)
Monosaccharides
Based on the number of carbon atoms in the sugar molecules
• Biose
• Triose
• Tetrose
• Pentose
• Hexose
• Heptose
Based on the functional groups
• Aldose
• Ketose
Oligosaccharides
2-10 number of monosaccharides
• Disaccharides: Sucrose àGlucose + fructose
• Trisaccharides: Raffinose àGlucose + Fructose + galactose
• Tetrasaccharides: Stachyose àGlucose + Fructose + 2 galactose
• Pentasaccharides: Verbascose
Polysaccharides
Indefinite number of monosaccharide units
• Homo polysaccharides: single type of monosaccharide
– Starch à glucose
– Cellulose à glucose
– Inulin
• Hetero polysaccharides: more than one type of monosaccharides
– Heparin
– Hyaluronic acid
Gums & Mucilage
Gums | Mucilage |
Pathological products | Physiological products |
Calcium, magnesium, potassium salts of complex substances, known as polyuronides | Sulphuric acid esters |
On boiling with dilute acids, they yield a mixture of sugars and uronic acids | Mixtures of neutral as well as acidic compounds |
Either hydrophobic or hydrophilic |
General chemical tests of carbohydrates
1. Fehling’s test
2. Molisch’s test
3. Barfoed’s test
4. Selvinoff’s test
5. Tollen’s test
6. Iodine test
7. Keller kiliani test
8. Ozozone test
MOLISCH TEST
Principle of Molisch Test:
• Carbohydrates when treated with concentrated sulphuric acid undergo dehydration to give furfural derivatives
• These compounds condense with Alpha naphthol to form colored products
• Pentoses yield furfural while Hexoses yield 5-Hydroxy methyl furfurals
Procedure of Molisch Test:
• Take 2 ml of carbohydrate solution in a clean and dry test tube
• Add 2 drops of ethanolic Alpha Naphthol (Molisch reagent) and mix
• Incline the test tube and add carefully 2 ml of concentrated sulphuric acid along the side of the test tube so as to form 2 layers
Interpretation of Molisch Test:
• An appearance of reddish violet or purple colored ring at the junction of two liquids is observed in a positive Molisch test
BENEDICT’S TEST
Principle of Benedict’s Test:
• Carbohydrates with free aldehyde or ketone groups have the ability to reduce solutions of various metallic ions
• Reducing sugars under alkaline conditions tautomerise and form enediols
• Enediols are powerful reducing agents
• They reduce cupric ions to cuprous form and are themselves converted to sugar acids
• The cuprous ions combine with OH- ions to form yellow cuprous
Procedure of Benedict’s Test:
• Take 5 ml of Benedict’s reagent, Add 8 drops of carbohydrate solution, Boil over a flame or in a boiling water bath for 2 minutes, Let the solution cool down
• Benedict‘s test is a semi quantitative test, color of the precipitate gives a rough estimate of a reducing sugar present in the sample
BARFOED’S TEST
Principle of Barfoed’s Test:
• Aldoses and ketoses can reduce cupric ions even in acidic conditions
• This test is used to distinguish reducing mono saccharides from disaccharides by controlling pH and time of heating
• Mono saccharides react very fast whereas disaccharides react very slowly
Procedure of Barfoed’s Test:
• To 2 ml of Barfoed‘s reagent, add 2 ml of carbohydrate solution, Keep the test tubes in the boiling water bath for 3 minutes, Cool under running water
Interpretation of Barfoed’s Test:
• The positive reaction indicates the presence of a reducing mono saccharide
• On prolonged heating disaccharides can also give this test positive
• Hence, the solution should be boiled for 3 minutes only
SELIIWANOFF’S TEST
Principle of Seliiwanoff’s Test:
• Keto hexoses on treatment with hydrochloric acid form
5-hydroxy methyl furfural which on condensation with resorcinol gives a cherry red colored complex
Procedure of Seliiwanoff’s Test:
• To 3 ml of Seliwanoff reagent add 1ml of fructose, Boil for 30 seconds only, cool the solution
Interpretation of Seliiwanoff’s Test:
• This test is given positive by ketohexoses so it is answered by fructose, sucrose and other fructose containing carbohydrates
• This test distinguishes between glucose and fructose.
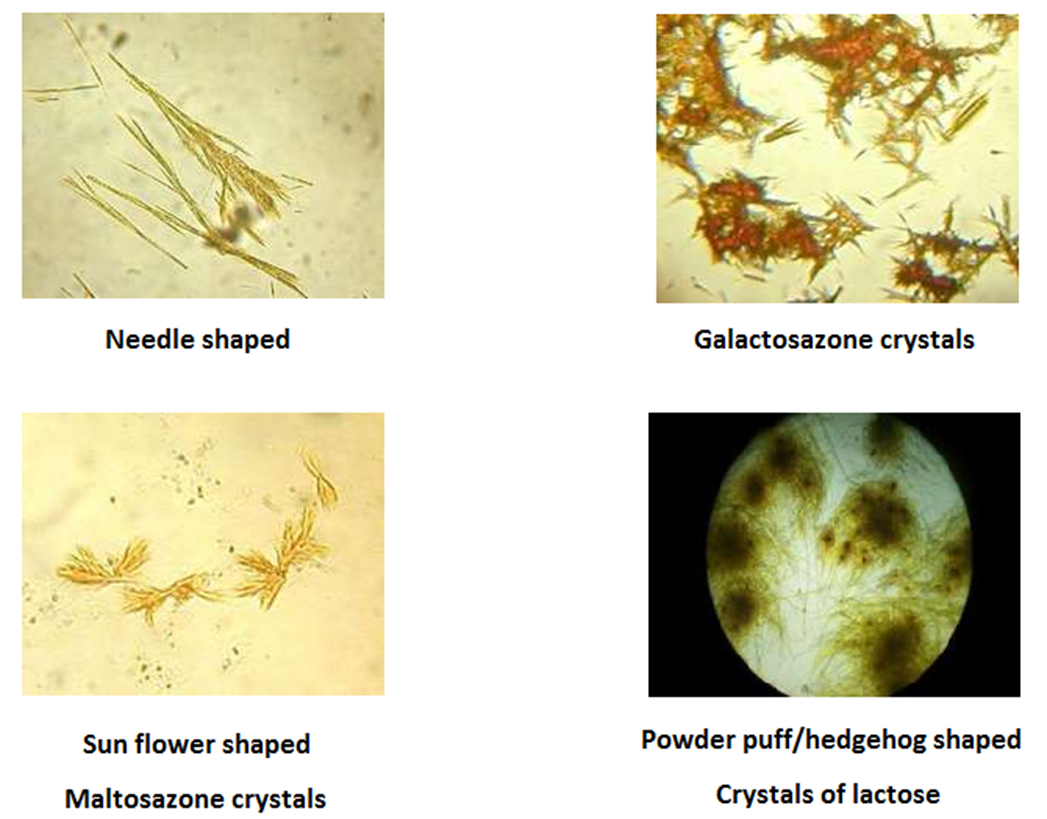
OSAZONE TEST
Principle of Osazone Test:
• A solution of reducing sugar when heated with phenyl hydrazine, characteristic yellow crystalline compounds called Osazone are formed.
• These crystals have definite crystalline structure, precipitation time and melting point for different reducing sugars
Procedure of Osazone Test:
• Add 10 drops of glacial acetic acid to 5 ml of sugar solution in test tube
• Then add a knife point of phenyl hydrazine hydrochloride and double the amount of sodium acetate crystals
• Mix and warm a little to see that the solids are dissolved
• Filter the solution in another test tube and keep the filtrate in a boiling water bath for 20 minutes
• Allow the tube to cool slowly in the water bath without cooling it hurriedly under the tap to have better crystals
• Examine the crystals under the microscope
BIAL’S TEST
Principle of Bial’s Test:
• The test reagent dehydrates pentoses to form furfural
• Furfural further reacts with orcinol and the iron ion present in the test reagent to produce a bluish product
Procedure of Bial’s Test:
• 2 ml of a sample solution is placed in a test tube
• 2 ml of Bial’s reagent (a solution of resorcinol, HCl and ferric chloride) is added
• The solution is then heated gently in a Bunsen Burner or hot water bath
• The formation of a bluish product
IODINE REACTION
• This is a test for polysaccharides
Principle Iodine Reaction:
• Iodine forms a coordinate complex between the helically coiled polysaccharide chain and iodine centrally located within the helix due to adsorption
• The color obtained depends upon the length of the unbranched or linear chain available for complex formation
Interpretation Iodine Reaction:
• Amylose- A linear chain component of starch, gives a deep blue color
• Amylopectin- A branched chain component of starch, gives a purple color
• Glycogen- Gives a reddish brown color Dextrins- Amylo, Eryhthro and Achrodextrins, formed as intermediates during hydrolysis of starch give violet, red and no color with iodine respectively
Summary
• Carbohydrates are polyhydroxy aldehydes or polyhydroxy ketones
• Classified into monosaccharides, oligosaccharides and polysaccharides
• Polysacchraides are classified into homo and hetero polysaccharides
• General chemical tests
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf