Chemistry of Purines
Session Objectives
By the end of this session, students will be able to:
• Discuss the chemistry, derivatives, properties and method
of synthesis of purine
PURINES AND PYRIMIDINES
• The word purine (pure urine) was coined by the German chemist Emil Fischer in 1884.
• He synthesized it for the first time in 1898.
• The starting material for the reaction sequence was uric acid.
• Uric acid was reacted with PCl5 to give 2,6,8trichloropurine, which was converted with HI and PH4I to give 2,6 diiodopurine .
• The product was reduced to purine using zinc dust.
• Nucleotides are the building blocks of nucleic acids
• Building blocks of nucleic acids (RNA, DNA) with certain
heterocyclic aromatic compounds called pyrimidines and purines.
• Purine and Pyrimidine are the names of the parent compounds of two types of nitrogen-containing heterocyclic aromatic compounds.
• A purine is a heterocyclic aromatic organic compound, consisting of a pyrimidine ring fused to an imidazole ring.
• Purines, including substituted purines and their tautomers, are the most widely distributed kind of nitrogen containing heterocycle in nature.
• Purines consist of a six-membered and a five-membered nitrogen-containing ring, fused together.
• The purine bases have a 9-membered double-ring system with four nitrogens and five carbons.
• Adenine and guanine are the principal purines of both DNA
and RNA.
Adenine (6-Aminopurine )
Guanine (2-Amino-6-oxypurine)
• The pyrimidine bases have a 6-membered ring with two
nitrogens and four carbons.
• Pyrimidines that occur in DNA are:
Cytosine and thymine.
Cytosine and uracil occur in RNA.
• Cytosine (2-oxy-4-amino pyrimidine)
• Thiamine (2,4-dioxy-5-methyl pyrimidine)
• Uracil (2,4-dioxy pyrimidine)
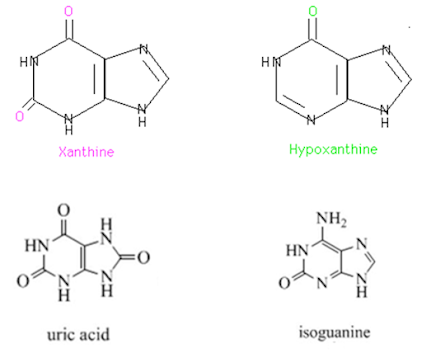
Naturally Occuring Purines
• Caffeine (coffee) and theobromine (coffee and tea) are
naturally occurring purines.
Synthesis of Purines
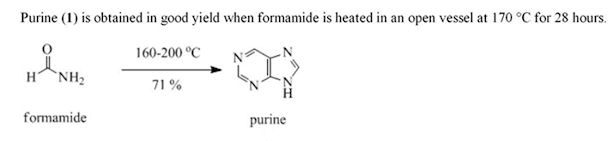
• Formamide (45 grams) was heated with a condenser for 28 hours in an oil bath at 170-190 °C.
• After removing excess formamide (32.1 grams) by vacuum
distillation, the residue was refluxed with methanol.
• The methanol solvent was filtered, the solvent removed
from the filtrate by vacuum distillation, and almost pure purine obtained; yield 4.93 grams (71% yield from formamide consumed).
• Crystallization from acetone afforded purine as colorless
crystals; melting point 218 °C
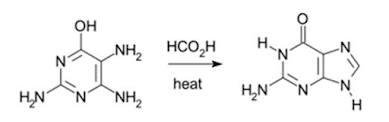
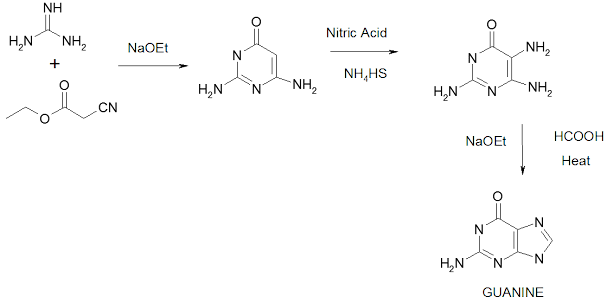
Traube purine synthesis
Synthesis of purines