Chemistry
of Imidazole & Oxazole
Session Objectives
By the end of this
session, students will be able to:
• Discuss the various method of synthesis of Imidazole and
Oxazole
• Discuss the chemistry, reactivity, properties of Imidazole
and Oxazole
Chemistry
of Imidazole
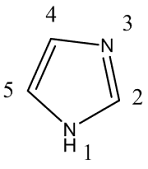
• Imidazole is isomeric with pyrazole or azapyrrole
• Also called as glyoxaline, as it was first prepared in
1858 from glyoxal and ammonia
• Imino nitrogen is assigned position-1 while tertiary
nitrogen atom position-3
• Imidiazole nucleus is found in number of naturally
occurring compounds such as histamine, histidine, pilocarpine and allantoin,
purine nucleus, vitamin B12
• Since imidazole also exists in tautomeric forms, either of
the nitrogen can bear the hydrogen atom and two nitrogen become
indistinguishable
• Numbering becomes rather complex for mono substitution
• For example, 4-methylimidazole is identical with
5-methylimidazole and depending on the position of imino hydrogen compound can
be designated
• Such compound is designated as 4(5)-methyl imidazole
Physical properties of Imidazole
• Imidazole is colorless liquid with boiling point 256 0C
and is high boiling point among all other five membered heterocyclic compounds
• Shows that hydrogen bonding exists in imidazole ring
• More basic with pka 7.2 than pyridine (pka 5.2)
Molecular
Properties of Imidazole
• Shows amphoteric properties and behaves as an acid because
it contains pyrrole type amino nitrogen in the ring
• Also forms metallic salts with NaNH2 and RMgX which are
extensively hydrolysed by water
• Introduction of alkyl groups increases the basicity,
2-methylimidazole (pka 7.86) and 4(5)-methyl imidazole (pka 7.52)
Synthetic
methods
1) Radiszewski
Synthesis: most important and common method
• Consists of condensing a dicarbonyl compound such as
glyoxal, α-keto aldehydes or α-diketones with aldehyde in the presence of
ammonia
• Benzil with benzaldehyde and two molecules of ammonia
react to yield 2,4,5-triphenylimidazole
2) Dehydrogenation of
imidazolines: can be prepared by dehydrogenation of imidazolines. Milder
reagent, barium manganate has been reported by Knapp and coworkers
• Imidazolines obtained from alkyl nitriles and
1,2-ethanediamine on reaction with BaMnO4 yields 2-substituted imidazole
Chemical
properties
1) Reaction with
acids: imidazole is a mono acidic base and forms crystalline salts with
acids
• Also possesses weakly acidic properties (pseudo acidic)
and is more acidic than pyrrole
• Forms salts with Grignard reagents or metal ions
2) Electrophilic substitution:
• Imidazole has increased reactivity toward electrophilic
attack and more susceptible than other five membered heterocycles
• From the resonance structures, electrophilic attack is
preferred at 4(5) position in imidazole
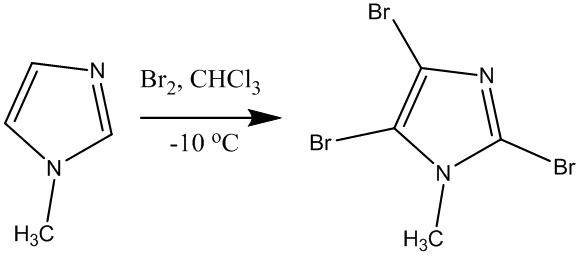
3) Halogenation:
very complex and depends upon type of substrate, reagents and reaction
conditions
• Bromination (Br2/CHCl3) yields 2,4,5-tribromoderivative
• Iodination (alkaline conditions) yields
2,4,5-triiodoimidazole
Chemistry
of Oxazole
• Oxazole is a 1,3-azole having an oxygen atom and a
pyridine type nitrogen atom at 3-position in a five membered ring
• First introduced by Hantzsch in 1887 but was not
synthesized until 1947
• Does not occur in nature and does not play any part in
fundamental metabolism like imidazole or thiazole
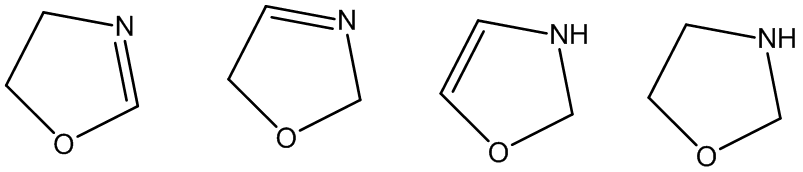
• Partially reduced oxazoles are called oxazolines
• Three types are possible depending on position of double
bond
• 2-oxazoline, 3-oxazoline and 4-oxazoline
• Fully saturated system is called oxazolidine- solids
Physical
properties of
• Oxazole is a liquid with boiling point 69 0C
• Odor resembling that of pyridine
• Miscible with water and many organic solvents
• Weakly basic (pka 0.8)
• Possess a sextet of π-electrons, delocalization is
incomplete and has little aromatic character
• Function as dienes in the Diels-Alder reaction and
electrophilic substitution is rare
Synthetic
methods
1) From ethyl
α-hydroxyl keto succinate: Bredereck and Bangert reported a simple method
which involves a reaction between ethyl α-hydroxyl keto succinate and formamide
to give diethyloxazole-4,5-dicarboxylate and subsequently hydrolysed and
decarboxylated to isoxazole
2) Robinson Gabriel
synthesis: most common method used for the synthesis of oxazoles
• Involves an α-acylamino ketone which undergoes cyclization
and dehydration in the presence of phosphorus pentoxide or strong mineral acid
• Applicable for the synthesis of 2,5-aryloxazoles
Chemical reactions
1) Electrophilic
substitution: preferred at 5th position of ring
• Occurs readily when ring is activated with electron
donating substituents
• Bromination (NBS) of 2-phenyloxazole results in
5-bromo-2-phenyloxazole
• Nitration and sulfonation of oxazoles are difficult
because of presence of pyridine type nitrogen
2) Diels-Alder
reaction: behaves similar to furan
• Introduction of second heteroatom does not effect the
diene nature of oxazole
Summary
• Imidazole is isomeric with pyrazole
• Imidazole also exists in tautomeric forms
• High boiling point- Shows that hydrogen bonding exists in
imidazole ring
• Shows amphoteric properties and behaves as an acid because
it contains pyrrole type amino nitrogen in the ring
• Imidazole has increased reactivity toward electrophilic
attack and more susceptible than other five membered heterocycles
• Oxazole is a 1,3-azole having an oxygen atom and a
pyridine type nitrogen atom at 3-position in a five membered ring
• Function as dienes in the Diels-Alder reaction and
electrophilic substitution is rare
• Electrophilic
substitution: preferred at 5th position of ring