Structure, Nomenclature
and Properties of Azepines
Session Objectives
By the end of this
session, students will be able to:
Discuss the chemistry, reactivity, properties and method of
synthesis of Azepines
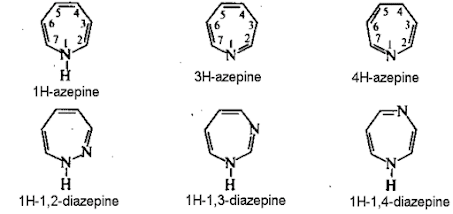
Azepines
• Seven
member heterocyclic ring compounds have received much attention in the past few
years owing to its wide range of biological activity.
• Azepines are heterocycles of seven atoms,
with a nitrogen replacing a carbon at one position.
A well
known azepine is caprolactam
• Skeletal
formula of caprolactam. ( Azepan-2-one)
• caprolactam
produced goes into the manufacture of Nylon 6.
• Caprolactam
is also used in the synthesis of several pharmaceutical drugs including
pentylenetetrazol, meptazinol, and laurocapram.
Seven member heterocycles which containing nitrogen
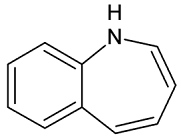
• Benzazepine: bicyclic structure consisting of
fused benzene and azepine rings; many compounds with this structure react with
biogenicamine receptors, and so are psychotropic and neurotropic.
Examples of benzazepines
Fenoldopam
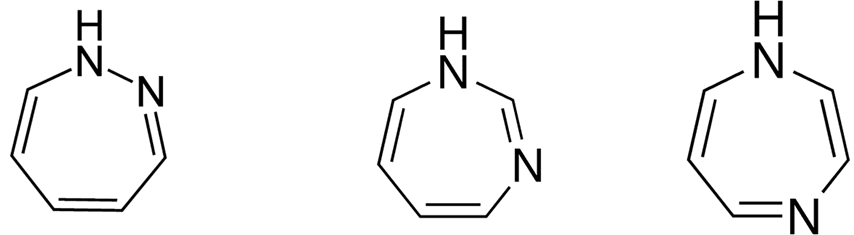
Structure, nomenclature and properties of diazepine. Benzodiazepine
• Diazepine is a seven member heterocyclic
compound with two nitrogen atoms (e.g., in ring positions 1 and 2) and three
double bonds.
• When diazepine
combined with a benzene ring, these is the basis of the benzodiazepine family .
In these compounds the nitrogen atoms are at the 1 and 4 positions as, for
example, in clobazam (depending on the position of the fused benzene ring, the
nitrogen atoms are also in positions number 1 and 4).
• The benzodiazepines
are a class of psychoactive drugs with varying hypnotic, sedative, anxiolytic
(anti-anxiety), anticonvulsant, muscle relaxant and amnesic properties, which
are mediated by slowing down the central nervous system. Benzodiazepines are
useful in treating anxiety, insomnia, agitation, seizures, and muscle spasms,
as well as alcohol withdrawal. They can also be used before certain medical
procedures such as endoscopies or dental work where tension and anxiety are
present, and prior to some unpleasant medical procedures in order to induce
sedation and amnesia for the procedure. Benzodiazepines are also used to treat
the panic that can be caused by hallucinogen intoxication.
• Benzodiazepines
can cause a physical dependence and a benzodiazepine addiction to develop and
upon cessation of long term use a benzodiazepine withdrawal syndrome can occur.
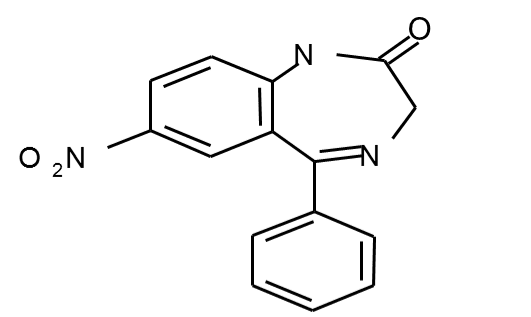
Nitrazepam
• Nitrazepam
is a nitrobenzodiazepine
• It is a
1,4 benzodiazepine, with the chemical name 7-nitro-5-phenyl-1,3-dihydro-1,4-
benzodiazepin-2-one.
• It is a
hypnotic drug with sedative and motor impairing properties, anxiolytic,
anticonvulsant and skeletal muscle relaxant properties. It is long acting drug,
has lipophilic and hepatometabolitic properties via oxidative pathways. It acts
on benzodiazepine receptors in the brain which are associated with the GABA
receptors (gamma-aminobutyric acid). GABA is a major inhibitor neurotransmitter
in the brain, involved in inducing sleepiness, muscular relaxation and control
of anxiety and seizures, and slows down the central nervous system.
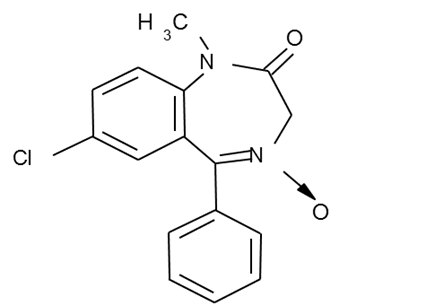
Diazepam
• (4N-oxide 7-chlorine-1-methyl-5-phenil-1,3-dihydro-2Н-1,4-benzodiazepin-2-оne)
• Diazepam first marketed as Valium by
Hoffmann-La Roche, is a benzodiazepine derivative drug. It possesses
anxiolytic, anticonvulsant, hypnotic, sedative, skeletal muscle relaxant and
amnestic properties. It is commonly used for treating anxiety, insomnia,
seizures, muscle spasms, alcohol withdrawal and benzodiazepine withdrawal.
• Diazepam
occurs as solid white or yellow crystals and has a melting point of 131.5 to
134.5 °C. It is odorless, and has a slightly bitter taste. The British
Pharmacopoeia lists diazepam as being very slightly soluble in water, soluble
in alcohol and freely soluble in chloroform. The United States Pharmacopoeia
lists diazepam as soluble 1:16 in ethyl alcohol, 1:2 in chloroform, 1:39 in
ether, and practically insoluble in water.
Qualitative reactions on benzodiazepines
1. With concentrated acids (H2SO4, HCl, HClO4) derivatives
of benzodiazepines form color salts.
2. Heterocyclic nitrogen atom gives positive reaction with
common alkaloids precipitate reagents.
3. Formation of azodyes after primary hydrolysis:
4. Noozepam by heating with conc. H2SO4 hydrolyzed with
formation of formaldehyde, which forms violet color with fuxinsulfite acid.