Conformational Isomerism in aliphatic systems

Conformational Isomerism in aliphatic systems
Session Objectives
By the end of this session, students will be able to:
• Explain the conformation of ethane
• Explain the conformation of butane
Conformational Isomerism in Aliphatic Systems
Conformational isomerism is a fundamental concept in organic chemistry, particularly in aliphatic systems. It plays a significant role in understanding the three-dimensional shapes and spatial arrangements of molecules. In this article, we will delve into the world of conformational isomerism, exploring its importance, types, energy differences, and real-world applications.
Types of Conformational Isomerism
Eclipsed Conformation
In the eclipsed conformation, the atoms in the molecule are as close as possible, leading to steric hindrance and higher energy. This conformation is less stable.
Staggered Conformation
In the staggered conformation, the atoms are as far apart as possible, resulting in lower energy and greater stability. This is the most favorable conformation.
Gauche Conformation
The gauche conformation is somewhat stable, with the atoms having a slight dihedral angle between them.
Anti Conformation
The anti conformation is highly stable, with the atoms being opposite to each other across the single sigma bond.
Energy Differences in Conformational Isomers
Conformational isomers have different energy levels. Eclipsed conformations have the highest energy, while staggered conformations have the lowest energy. This energy difference is crucial in understanding the stability and reactivity of molecules.
Factors Influencing Conformational Stability
Several factors influence the stability of conformational isomers:
Steric Hindrance
Steric hindrance occurs when bulky groups in a molecule come too close to each other, leading to repulsive forces and higher energy.
Hyperconjugation
Hyperconjugation involves the delocalization of electrons from sigma bonds to adjacent empty p-orbitals, stabilizing the molecule.
Dipole-Dipole Interactions
Dipole-dipole interactions can either stabilize or destabilize a conformation, depending on the relative orientation of dipoles.
Why staggered conformation of ethane is lower in energy than the eclipsed conformation?
• Steric interaction between hydrogen atoms in the eclipsed conformation
• The hydrogen atoms are too small to get in each other’s way
• It has been estimated that steric factors make up less than 10% of the rotational barrier in ethane
• Can be justified with two important reasons
1) The first is that the electrons in the bonds repel each other and this repulsion is at a maximum in the eclipsed conformation
2) The second is that there may be some stabilizing interaction between the C–H σ bonding orbital on one carbon and the C–H σ* anti-bonding orbital on the other carbon, which is greatest when the two orbitals are exactly parallel: this only happens in the staggered conformation.
Ethane Conformations
• The torsional energy of ethane is lowest in the staggered conformation.
• The eclipsed conformation is about 3.0 kcal/mol (12.6 kJ/mol) higher in energy.
• At room temperature, this barrier is easily overcome, and the molecule rotate constantly.
Conformational Isomerism Definitions
Eclipsed conformation: A conformation about a carbon-carbon single bond in which the atoms or groups on one carbon are as close as possible to the atoms or groups on an adjacent carbon
Staggered conformation: A conformation about a carbon-carbon single bond in which the atoms or groups on one carbon are as far as possible to the atoms or groups on an adjacent carbon
Dihedral angle: An angle created by two intersecting planes
Anti-conformation: A conformation about a single bond in which two groups on adjacent carbons lie at a dihedral angle of 1800
Gauche conformation: A conformation about a single bond of an alkane in which two groups of adjacent carbons lie at a dihedral angle of 600
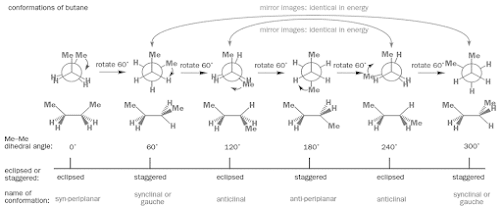
Conformations of butane
• Coming to butane, structure is slightly complicated
• We have replaced two hydrogen atoms in ethane by larger methyl groups
• Steric factors become a significant contribution to the rotational energy barriers.
• Also when we rotate C-C bond, not all the staggered conformations are same, neither are all the eclipsed conformations
• The six conformations butane can adopt as the central C-C bond is rotated in 600 is explained in the next slide
Visualization of Conformers of butane
Energy relationship of conformers
• If we look those conformers, can observe the conformations with dihedral angles 600 and 3000 and with angles 1200 and 2400 are mirror images of each other
• Means we have only four different maxima or minima in energy on rotation of central C-C bond
• In the four, two types of eclipsed conformations which represents the maxima in energy rotation graph and two types of staggered conformations which represents the minima
• All the conformations have been explained with respective names
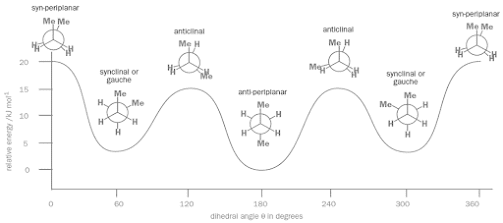
Relationship between energy and dihedral angle
Observations in the graph
• Each of the eclipsed conformations will be energy maxima but the syn-periplanar conformation (θ = 0°) will be higher in energy than the two anticlinal conformations (θ = 120° and 240°)
• In the syn-periplanar conformation two methyl groups are eclipsing each other whereas in the anticlinal conformations each methyl group is eclipsing only a hydrogen atom
• The staggered conformations will be energy minima but the two methyl groups are farthest from each other in the anti-periplanar conformation
• Anti-periplanar will be a slightly lower minimum than the two synclinal (gauche) conformations
• Staggered conformations are stable since they each lie in a potential energy well
• Anti-periplanar conformation, with the two methyl groups opposite each other, is the most stable of all
• Eclipsed conformations are energy maxima, and therefore represent the transition states for interconversion between conformers
Summary
• Steric interaction between hydrogen atoms in the eclipsed conformation
• Six conformations butane can adopt as the central C-C bond is rotated in 600
• Eclipsed conformations are energy maxima
Frequently Asked Questions
- What is the difference between conformational isomerism and structural isomerism?
- Conformational isomerism is related to the rotation of sigma bonds, while structural isomerism involves different connectivity of atoms in a molecule.
- Why are staggered conformations more stable than eclipsed conformations?
- Staggered conformations have lower energy because the atoms are as far apart as possible, reducing steric hindrance.
- How is conformational energy measured?
- Conformational energy is measured using computational methods and spectroscopy, which help determine the energy associated with each conformation.
- Are conformational isomers relevant in real-world applications?
- Yes, conformational isomerism is relevant in industries like petrochemicals and pharmaceuticals, where it affects properties like boiling and melting points.
- Can conformational isomerism be applied in drug design?
- Yes, understanding conformational isomerism is crucial in drug design to optimize the effectiveness and safety of pharmaceutical compounds.
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf