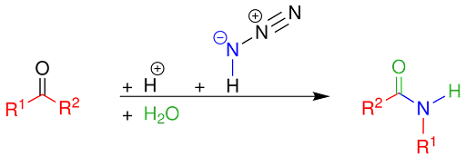Schmidt Rearrangement
Session Objectives
By the end of this
session, students will be able to:
• Steps involved in Schmidt rearrangement
• Reaction Mechanism of Schmidt rearrangement
Schmidt Rearrangement
• Schmidt reaction is an organic reaction in which an azide
reacts with a carbonyl group to give an amine or amide, with expulsion of nitrogen.
• It is named after Karl Friedrich Schmidt (1887–1971), who
first reported it in 1924 by successfully converting benzophenone and hydrazoic
acid to benzanilide.
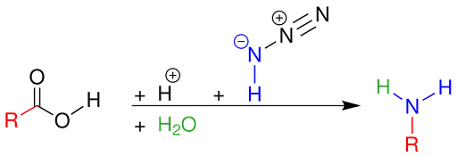
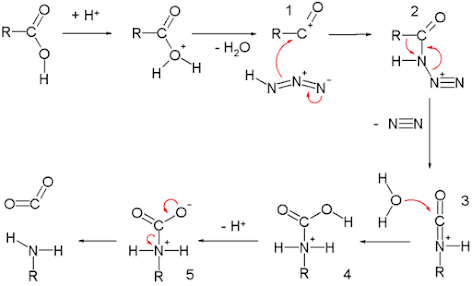
Reaction Mechanism for
carboxylic acid Schmidt reaction
The carboxylic acid Schmidt reaction starts with acylium ion
1 obtained from protonation and loss of water.
Reaction with hydrazoic acid forms the protonated azido
ketone 2, which goes through a rearrangement reaction with the alkyl group R,
migrating over the C-N bond with expulsion of nitrogen.
The protonated isocyanate is attacked by water forming
carbamate 4, which after deprotonation loses carbon dioxide to the amine.
Reaction Mechanism for Ketone
Schmidt reaction
In the reaction mechanism for the ketone Schmidt reaction,
the carbonyl group is activated by protonation for nucleophilic addition by the
azide, forming intermediate 3, which loses water in an elimination reaction to
temporary imine 4, over which one of the alkyl groups migrates from carbon to nitrogen
with loss of nitrogen.
A similar migration
is found in the Beckmann rearrangement. Attack by water and proton loss
converts 5 to 7, which is a tautomer of the final amide.
Summary
• Schmidt reaction is an organic reaction in which an azide
reacts with a carbonyl group to give an amine or amide, with expulsion of
nitrogen.
• It is named after Karl Friedrich Schmidt (1887–1971), who
first reported it in 1924 by successfully converting benzophenone and hydrazoic
acid to benzanilide.