Single Cell Culture
Objective
At the end of the lecture the student will able to:
• Discuss the isolation of single cell
• Identify the suitable method for culturing single cell
Single Cell Culture
• Single cell culture is a method of growing isolated single
cell aseptically on a nutrient medium under controlled condition
• Basic principle – isolation of large number of intact
living cells and culture them on a suitable nutrient medium
• Single cells can be isolated from a variety of tissue and
organ of green plant as well as from callus tissue and cell suspension
• Single cells from the intact plant tissue (leaf, stem,
root etc.) are isolated either mechanically or enzymatically
Mechanical isolation:
• Tearing or chopping the surface sterilized explant to
expose the cells followed by scraping of the cells with a fine scalpel to liberate the single cell
• But very few living cells are obtained for a lot of time
and effort
• Gentle grinding of surface sterilized explant in a
sterilized mortar- pestle followed by cleaning the cells by filtration and centrifugation is now widely used
Enzymatic isolation:
• More efficient way of large-scale isolation of free cells
from the surface sterilized is to dissolve the intercellular cementing material, i.e. pectin, by pectinase or macerozyme treatment
• Enzyme macerates the tissue from which large-number of
variable cells can be obtained
• The special feature of enzymatic isolation of cell is that
it has been possible to obtain pure preparation of viable cells with less effort and time
Isolation from callus
and suspension culture cells:
• Mechanically, single cells are carefully isolated from
cell suspension or friable callus with a needle or fine glass capillary
• Alternatively, the friable tissue is transferred to liquid
medium and the medium is continuously agitated by a shaker
• Agitation of liquid medium breaks and dispenses the single
cells and cell clumps in the medium- cell suspension
• Cell suspension is first filtered to remove cell clumps,
filtrate is then centrifuged to collect the single cells from the pellet
Methods of single cell culture:
• Paper raft nurse technique
• Petri dish plating technique
• Micro-chamber technique
• Plating with nurse tissue technique
• Micro-droplet technique
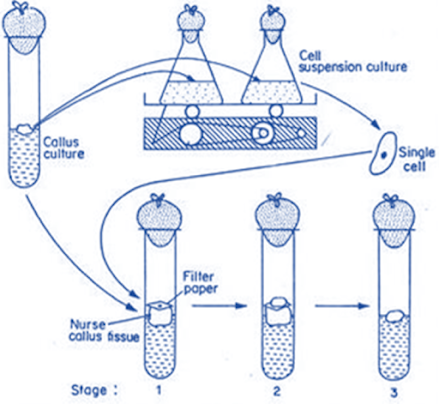
Paper raft nurse technique
• Single cells are isolated from suspension cultures or a
friable callus with the help of a micropipette or micro-spatula
• Few days before cell isolation, sterile (8 x 8 mm) squares
of filter paper are placed aseptically on the upper surface of the actively growing callus tissue of the same or different species
• The filter paper will be wetted by soaking in the water
and absorbs nutrient from the callus tissue
• Isolated single cell is placed aseptically on the wet
filter paper raft
• The whole culture system is incubated under 16 hrs, cool
under continuous darkness at 25° C
• Single cell divides and re-divides and ultimately forms a
small cell colony
• When the cell colony reaches a suitable size, it is
transferred to fresh medium where it gives rise to the callus tissue
• Callus tissue, on which the single cell is growing, is
called the nurse tissue
• Nurse tissue supplies nutrients to the cell from the
culture medium and also helps in cell division
• Single cell absorbs nutrients through filter paper,
nutrients actually diffuse upward from culture medium through callus tissue andbfilter paper to the single cell
• A callus tissue originating from a single cell is known as
a single cell clone
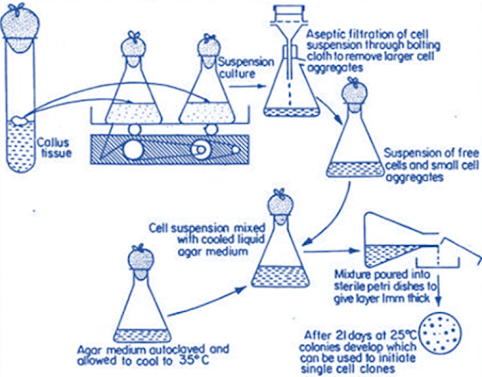
Petri dish platingntechnique
• A suspension of purely single cells is prepared
aseptically from the stock cell suspension culture by filtering and centrifugation
• Solid medium (1.6% ‘Difco’ agar added) is melted in water
bath
• In front of laminar air flow, the tight lid of falconnplastic petridish is opened with the help of sterilized Pasteur pipette
• 1. 5 ml of single cell suspension is put an equal amount
of melted agar medium when it cools down at 35°C, is added in the single cell suspension
• Lid is quickly replaced and the whole dish is swirled gently to disperse the cell and medium mixture uniformly
• Medium is allowed to solidify and the petri dish is kept
at the inverted position
• Cultures are incubated under 16hrs light or under
continuous dark at 25°C.
• Petri dishes are observed at regular intervals under
inverted microscope to see whether the cells have divided or not.
• After incubation, when the cells start to divide, a grid
is drawn on the under surface of the petridish to facilitate counting thebnumber of dividing cells
• Dividing cells ultimately form pin-head shaped cell colonies
within 21 days of incubation
• Plating efficiency (PE) can be calculated by the following
formula
Number of colonies per plate/Number of total cell per plate x 100
• Colonies, when they reach a suitable size, transferred to
fresh medium for further growth
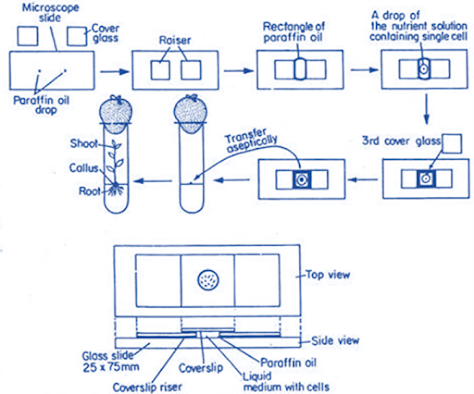
Micro-chamber Technique
• A drop of liquid nutrient medium containing single cell is
first isolated aseptically from stock suspension culture with the help of long fine Pasteur pipette.
• The culture drop is placed on the centre of a ‘sterile
microscopic slide (25 x 75 mm) and ringed with sterile paraffin oil.
• A drop of paraffin oil is placed on either side of the
culture drop and a cover-glass (raiser) is placed on each oil drop
• A third cover-glass is then placed on the ‘culture drop
bridging the two raiser cover-glasses and forming a micro-chamber to enclose the single cell aseptically within the paraffin oil
• Whole micro-chamber slide is placed in a petridish,
incubated under white light (3,000 lux) at 25 0C for 16 hrs
• When the cell colony becomes sufficiently ‘large, the
cover-glass is removed and the tissue is transferred to fresh medium
• Micro-chamber technique permits regular observation of the growing and dividing cell
Plating with nurse tissue technique
• Modification of petridish plating method and the paper
raft nurse culture method.
• In this method, single cells are plated on to agar medium in
a petridish
• Two or three callus masses (Nurse Tissue) derived from the same plant tissue are also embedded directly along with the single cells in the same medium
• Here the paper barrier between single cells and the nurse
tissue is removed
• Cells first begin to divide in the regions near the nurse
callus
• indicates that the single cells closer to nurse callus in
the solid medium gets the essential growth factors
• The developing colonies growing near to nurse callus also
stimulate the division and colony formation of other cells
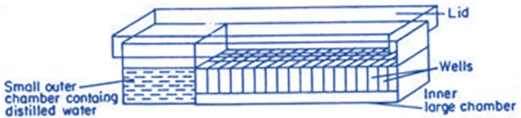
Micro drop technique
• Single cells are cultured in special Cuprak dishes which
have two chambers, a small outer chamber and a large inner chamber
• Large chamber carries numerous numbered wells each with a capacity of 0.25-25µl of nutrient medium
• Each well of the inner chamber is filled with a micro-drop
of liquid medium containing isolated single cell
• Outer chamber is filled with sterile distilled water to
maintain the humidity inside the dish
• After covering the dish with lid, the dish is sealed with
paraffin
• Dish is incubated under 16hrs cool light (3,000 lux) at
25°C
• Cell colony derived from the single cell is transferred on
to fresh solid or semisolid medium in a culture tube for further growth
Application of Single Cell Culture
1. Micro Propagation
2. Clonal Propagation
3. Production of Genetically Variable Plants
4. Plant Pathology and Plant Tissue Culture
5. Plant Breeding, Plant Improvement and Plant Tissue Culture
6. Production of Useful Bio-chemicals
7. Preservation of Plant Genetic Resources or Gene
Conservation Bank
8. Importance of Tissue Culture in Biotechnology
Summary
• Single cell culture is a method of growing isolated single
cell aseptically on a nutrient medium under controlled condition
• Single cells – intact plant tissue callus tissue and cell
suspension
• Single cells – Mechanically or enzymatic method
• Paper raft nurse, Petri dish plating, Micro-chamber,
Plating with nurse tissue and Micro-droplet technique
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf