Stereochemistry of Cyclohexane
Session Objectives
By the end of this
session, students will be able to:
• Explain Ring inversion of cyclohexane
• Discuss factors effecting mono and disubstitution of
cyclohexane with examples
Drawing Cyclohexane
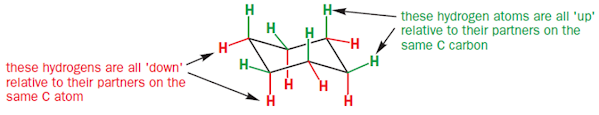
• In the structure of cyclohexane, all the six carbons are
identical but there are two types of hydrogens
• One type stick either vertically up or down are called
axial hydrogen atoms
• Others stick out sideways are called equatorial hydrogen
atoms
• Each carbon is attached to two hydrogens, one in axial and
other in equatorial positions
Ring inversion or flipping of cyclohexane
• Chair conformer is the preferred conformation for
cyclohexane
• 13CNMR shows single signal showing that all six
carbons are same
• But there are two different sorts of protons- axial and
equatorial. Still 1HNMR shows only one signal
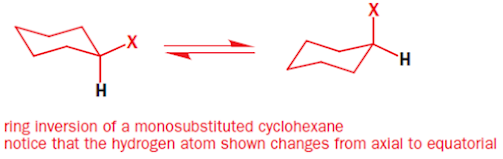
• In monosubstituted cyclohexane, there should be two
isomers detectable one with axial and the other with equatorial
• But at room temperature again one signal is seen
• This gives us a clue that two isomers are conformers and
interconvert rapidly at room temperature called as ring inversion or flip
• After ring inversion, all the bonds in axial changes to
equatorial and vice versa
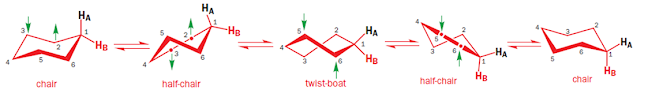
Ring inversion process
• The whole inversion process can be broken down into
conformations shown below
• The green arrows show the direction in which the
individual carbon atoms should move in order to get to the next conformation
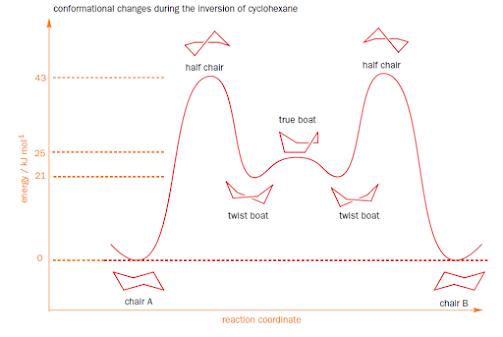
Energy profile for ring inversion of cyclohexane
• Shows that half-chair is the energy maximum in the
conversion of chair to twist boat
• True boat conformation is the energy maximum on
interchanging between two mirror image twist boat conformers
• The second twist boat is converted to other chair
conformation through another half-chair
• This clearly shows that ring inversion interconverts the
axial and equatorial protons too fast for them to be detected by NMR
• Exchanging occurs at a rate of 2 x 105 s-1
at 25 0C
Substituted cyclohexane- mono
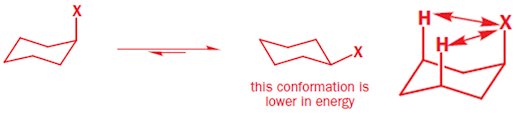
• Cyclohexane is free of angle strain, torsional strain and
van der waals strain
• In monosubstitution, there can exist two different chair
conformers- one with axial and other with equatorial
• Both will be in equilibrium, but of different energies
with axial substituent in higher energy (7.3 KJ mol-1 higher than
equatorial)
• In cyclohexane, a given atom or group has more room in an
equatorial position than in an axial position
• Why axial is higher in energy than equatorial conformer?
1) axial conformer is destabilized by repulsion between the
axial group X and two axial hydrogen atoms on the same side of the ring known
1,3-diaxial interaction. Increases with X gets larger
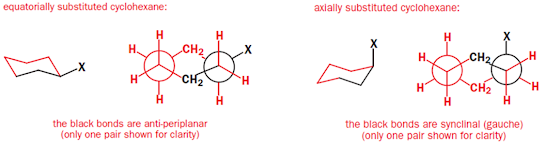
2) In the equatorial conformer the C–X bond is
anti-periplanar to two C–C bonds, while, for the axial conformer, the C–X bond
is synclinal (gauche) to two C–C bonds
• The amount of equatorial conformer present does increase
in the order Me < Et < i-Pr < t-Bu
• The equilibrium constant does not depend on the actual
size of the substituent, but rather its interaction with the neighbouring axial
hydrogens
• In the case of the methoxy group, the oxygen acts as link
and removes the methyl group away from the ring, lessening the interaction-
methoxy group prefers axial
What happens with more than one substitution on the ring?
• When there are two or more substituents on the ring,
stereoisomerism is possible
• For example, 1,4-cyclohexanediol, two isomers are
possible- cis isomer (both the substituents are either above or below ring),
trans isomer (one is above and other is below)
• For a cis-1,4-disubstituted cyclohexane with both
the substituents same, ring inversion leads to a second identical conformation
• For the trans configuration there is one
conformation with both groups axial and one with both groups equatorial
Disubstitution on cyclohexane
Summary
• Chair conformer is the preferred conformation for
cyclohexane
• Ring inversion interconverts the axial and equatorial
protons too fast for them to be detected by NMR
• Cyclohexane is free of angle strain, torsional strain and
van der waals strain
• In the disubstitution of cyclohexane, we need to consider
the following factors:
1) Position isomerism
2) Stereoisomerism which includes geometrical and optical
3) Relative sizes of the two substituents
4) Nature of the substituents