Free Radical Substitution Reactions of Alkanes/Halogenation of Alkanes
Contents
• Substitution reaction
• Free radicals
• Chain initiation
• Chain propagation
• Chain termination
• Uses of paraffins
Learning
Objectives
At the end of this lecture, student will be able to
• Explain the mechanism involved in free radical
substitution reactions of alkanes
Alkanes or Paraffins
• Alkanes are
also known as saturated hydrocarbons and sometimes as paraffins
• The name
“paraffin” is based on the Latin words parum + affinis =
“little affinity”.
• The
“little affinity” behind their Latin name referring to their
relatively low reactivity.
• They have
“little affinity” for other elements or compounds
Uses of alkanes
• Methane.
The largest amount of methane produced is used for heating purposes
• Ethane is
being used as heating fuel
• Propane
is an important raw material in petrochemistry
• Butane is
an important feedstock for the petrochemical industry
Properties of alkanes
• Saturated hydrocarbons
• Contains only carbon-carbon single bond
• C-H bond- non-polar covalent bond
• Alkanes- non-polar compounds
• Quite unreactive- contain only strong sigma bonds
• A compound containing a halogen atom covalently bonded to
an sp3 hybridized carbon atom. Given the symbol R-X
• Called as haloalkane or alkyl halide
Preparation of haloalkanes
• Halogenation of alkanes
• Can also be prepared by addition of X2 to
alkenes
• Halogenation of alkanes is common with Br2 and
Cl2
• Mixture of methane and chlorine gas- in dark and at room
temperature- no detectable change
• On heating or exposed to light- reaction occurs
• If allowed to react with more amount of chlorine
• Regioselectivity
• Treating propane with bromine gives a mixture consisting
of approximately 92% of 2-bromopropane and 8% of 1-bromopropane
Problem-01
• Bromination
Free
Radical Substitution Reaction of Alkanes
• Chlorination of methane
Step 1
Mechanism of Halogenation of Alkanes
• Occurs as radical chain mechanism
• Involve three types of steps
1) Chain
initiation
2) Chain
propagation
3) Chain
termination
Mechanism of Free Radical Substitution Reaction
Step 1
• Chain Initiation step
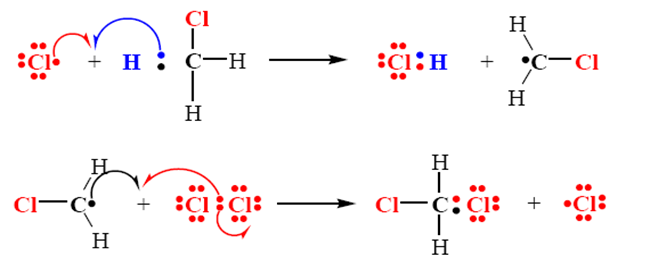
Step 2
• Chain propagation
step
Step 3
• Chain propagation
step
Step 4
• Chain termination
step
– coupling of 2 free radicals
• Chain propagation
step
– Formation of CH2Cl2
Summary
• Alkanes are also known as saturated hydrocarbons and
sometimes as paraffins
• The name “paraffin” is based on the Latin words
parum + affinis = “little affinity”.
• The “little affinity” behind their Latin name referring to
their relatively low reactivity.
• They have “little affinity” for other elements
or compounds
• Methane. The largest amount of methane produced is used
for heating purposes
• Ethane is being used as heating fuel
• Propane is an important raw material in petrochemistry
• Butane is an important feedstock for the petrochemical
industry
• Free radicals – highly reactive reaction intermediates
with odd electron formed by homolysis of covalent bond
• Free radicals – generated by heat or irradiation of light
or initiators
• Halogenation of alkanes takes place by free radical chain
reactions
• Free radical mechanism involves 3 steps – chain
initiation, propagation and termination
• Free radicals – generated in the initiation step
• One radical generates another radical with the formation
of product in the propagation step
• Chain termination takes place by coupling of 2 free
radicals
• Butane is an important feedstock for the petrochemical
industry