Hybridisation
Contents
• Hybridisation
• SP2
• Sp3
• Bong angles
• Geometry
Learning
Objectives
At the end of this
lecture, student will be able to
• Explain different types of hybridisation
• Relate hybridisation to geometry of molecules
Hybridisation
• Hybrid orbital- An orbital formed by the combination of
two or more atomic orbitals
• Hybridization- The combination of atomic orbitals of
different types
• Number of hybrid orbitals formed is equal to the number of
atomic orbitals combined
• sp3 Hybrid Orbitals—Bond Angles of Approximately 109.5°
• Combination of the 2s atomic orbital and three 2p atomic
orbitals forms four equivalent sp3 hybrid orbitals
• Mixing of orbitals
• To attain equal energy and stability
• Sp3
• Sp2
• Sp
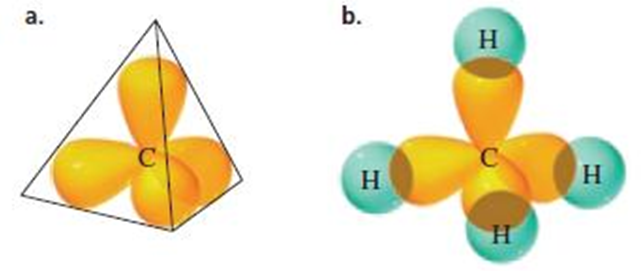
Sp3
Hybridisation
• Axes of
the four sp3 hybrid orbitals are directed toward the corners of a
regular tetrahedron
• sp3 hybridization results in bond angles
of approximately 109.5°
• Each sp3
orbital has 25% s-character and 75% p-character
• Because
those are the percentages of the orbitals combined when constructing them
• one 2s
orbital, three 2p orbitals
The four orbitals are directed toward the corners of a
tetrahedron, causing each bond angle to be 109.5°
Sp2
Hybridisation
• Bond
angles of approximately 1200
• Combination
of one 2s atomic orbital wave function and two 2p atomic orbital
• Forms
three equivalent sp2 hybrid orbital
• Axes of
the three sp2 hybrid orbitals lie in a plane and are directed toward the
corners of an equilateral triangle
• Three
equivalent sp2 orbitals along with the remaining unhybridized 2p atomic
orbital
• Each sp2 orbital has 33% s-character and 67% p-character
• One 2s orbital, two 2p orbitals
Borane
(BH3)
• Sp2
hybridization
• Exception
to octet rule
• According
VSEPR theory, trigonal planar with bond angle 1200
Trigonal planar carbon, 120o
sp hybridization
• Bond
angle of approximately 1800
• Combination
of one 2s atomic orbital and one 2p atomic orbital
• Produces
two equivalent sp hybrid orbital
• Axes of the
unhybridized 2p atomic orbitals are perpendicular to each other and to
the axis of the two sp hybrid orbitals
• Each sp
orbital has 50% s-character and 50% p-character
Summary
• Sp3, sp2 and sp
hybridisation
• Example for Sp3 hybridisation is methane and ethane
• Four Sp3 hybridized orbitals
• All bonds are sigma bonds
• Geometry of methane is tetrahedral and bond angle is
109.5°
• Example for sp2 hybridisation is ethene
• Three Sp2 hybridized orbitals
• One unhybridised p- orbital oriented perpendicular to the
plane
• One π bond is formed
• Geometry of ethene is trigonal and bong angle is 120°
• Example
for sp hybridisation is ethyne
• Two Sp
hybridized orbitals
• Two
unhybridised P- orbitals oriented pependicular to the plane
• Two π-bonds are formed
• Geometry
of ethyne is linear and bond angle is 180°