Adrenergic agents
Intended
learning outcomes
At the end of the
lecture students will be able to
• Classify the nervous system
• Understand the biosynthesis of adrenaline
• Predict the metabolic products of adrenaline
• Describe the effects of the neurotransmitter on the
various receptors
• Definition of sympathomimetic drugs
• SAR of sympathomimetic drugs
Divisions
of human nervous system
Human Nervous system
1. Central Nervous System
2. Peripheral Nervous System
– Autonomic Nervous System
Peripheral Nervous
System
Peripheral Nervous System
– Includes neurons and ganglia outside of the brain and spinal cord
1. *Autonomic Nervous
System (involuntary) *Either “fight and flight” mode or “rest and digest”
2. Somatic Nervous
System (voluntary)
– Sympathetic Nervous System (adrenergic)
– Parasympathetic Nervous System (cholinergic)
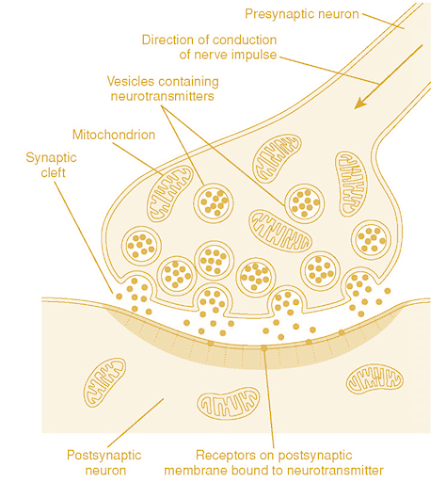
Neurotransmission
Adrenergic
nervous system:
• Adrenergic nervous system is a group of organs and nerves
in which adrenaline and/or noradrenaline are released as neurotransmitters
• Adrenergic nerve release neurotransmitters: noradrenaline,
adrenaline, dopamine and produce their effect
Adrenergic
transmission
Catecholamine:
• Natural: Adrenaline, Noradrenaline, Dopamine
• Synthetic: Isoprenaline, Dobutamine
• Non-Catecholamines:– Ephedrine, Amphetamines,
Phenylepherine, Methoxamine, Mephentermine
• Also called sympathomimetic amines as most of them contain
an intact or partially substituted amino (NH2) group
• Noradrenaline/
norepinephrine: It is transmitter at postganglionic sympathetic sites
(except sweat glands, hair follicles and some vasodilator fibres)
•
Adrenaline/Epinephrine: It is secreted by adrenal medulla and may have a
transmitter role in the brain
• Dopamine: it is
a major transmitter in basal ganglia, limibic system, CTZ, anterior pituitary,
etc
Biosynthesis
of Catecholamine
• NE is synthesized, stored and released in the synaptic
vessels of the sympathetic neurons.
• Adrenaline is synthesized and stored in the adrenal
medulla and released only in emergency conditions.
Step 1:
• L-Tyrosine is transported actively into the adrenergic
neuron, where it is 3’-hydroxylated by tyrosine hydroxylase (TH, tyrosine
-3-monooxygenase).
• L-DOPA = dihydroxyphenylalanine.
• Enzyme requires molecular O2, Fe2+,
and a tetrahydropteridine cofactor.
• Enzyme follows end-product inhibition, feedback
inhibition.
Step 2:
• Decarboxylation of L-DOPA to give DA by enzyme DOPA
decarboxylase (L-aromatic amino acid decarboxylase).
Step 3:
• DA is actively transported into storage vesicles by
vesicular monoamine transporter (VMAT).
• Side-chain hydroxylation of DA gives NE by dopamine β
-hydroxylase (DBH, dopamine β -monooxygenase).
Step 4:
• N-methylation of NE to give E in the adrenal medulla by
phenylethanolamine-N-methyltransferase (PNMT).
• PNMT is a cytosolic enzyme and the methyl donor S-adenosyl methionine (SAM) is required
for the N-methylation of NE.
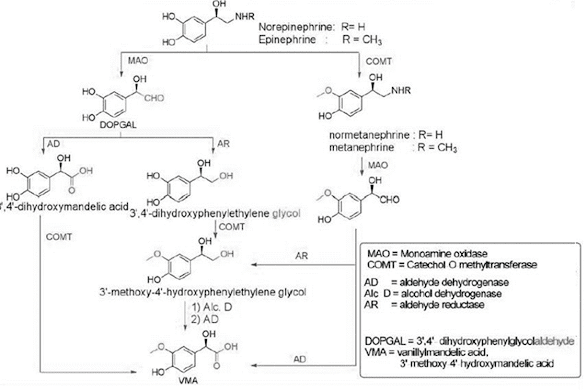
Metabolism of Catecholamine
• These metabolites are conjugated with glucuronides and
sulphates and gets eliminated
Adrenergic
receptors
Adrenergic Receptor
Subtypes
• Are membrane-associated G-protein-coupled receptors.
• G-protein = Guanine nucleotide-binding proteins.
• In 1948, Ahlquist proposed and designated α-
and β- adrenoceptors based on their apparent drug sensitivity.
• Further division of adrenoceptors α1A, α1B, α1D, α2A, α2B,
α2C, β1, β2, β3.
• Imidazolines show high affinity
toward α2-adr. Receptor, thus is also called imidazoline receptor.
Sympathomimetic
agents
• Compounds that produce effects similar to stimulation of
sympathetic nervous system activity are known as sympathomimetic.
Synonyms:
Adrenergic Stimulants
Act by: stimulating
adrenergic receptors or affect the life cycle of adrenergic neurotransmitters.
SAR of
Sympathomimetic agents
• The common structural features required for adrenergic
agents are asubstituted benzene ring and a primary or secondary aliphatic
aminogroup separated by 2 carbon atoms from benzene ring.
• The agent in this class have a hydroxyl group on the
β-carbonatom of the side chain.
• Hydroxy substituted carbon must be in R absolute configurationfor
maximum direct activity.
Substitution on the
amino group
• The receptor selectivity depends on the size of the alkyl
group present on the nitrogen atom.
• Increase in the size from hydrogen in nor-adrenaline to
isoproterenol decreases activity at α-receptor and increases activity at
β-receptor.
• Substitution of amino group with a tertiary butyl group
also provide selectivity for different β receptors.
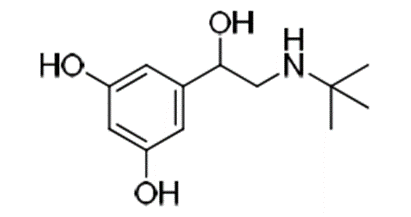
e.g. Terbutaline is a
selective β2 agonist whereas, isoprenaline is a non-selective β agonist.
Terbutaline
Substitution on the α-carbon
atom in the side chain
• Small alkyl groups like methyl or ethyl may be present on the-
carbon atom. Such substitution slow the metabolism
carried out by Mono amine oxidase.
• An ethyl group in this position diminish α-activity and
afford compound having β activity.
• Substitution on this carbon introduce another asymmetric center
producing pairs of diastereomers, which can have significantly different
biological activity.
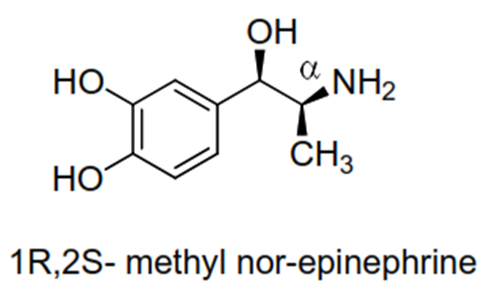
• e.g. maximum direct activity in streoisomer of α-methyl nor-
epinephrine reside with the streoisomer having 1R, 2S absolute configuration.
While 1R, 2R streoisomer is indirectly activity.
Substitution on the
aromatic ring
• Compound having both 3,4-dihydroxy group on benzene ring
are active at both α and β receptors and they rapidly metabolize COMT.
• Change in substitution pattern to 3,5-dihydroxy as in
terbutaline gives good oral activity and selectivity for β2 receptor.
Terbutaline
Summary
• Adrenaline or epinephrine is the neurotransmitter of the
adrenergic nervous system
• It is also called as the sympathetic nervous system
– It comes under the autonomic nervous system
• It is synthesized in the body from the amino acid tyrosine
• It is metabolized by MAO and COMT, and finally eliminated
after conjugation with gluconoride
• The five main categories of adrenergic receptors are: α1,
α2, β1, β2, and β3.