Aliphatic Nucleophilic Substitution Reaction
Contents
• Aliphatic substitution reaction
• Nucleophile
• Leaving group
• Bimolecular substitution reaction
• Mechanism
• Factors affecting rate of reaction
• Structure of the substrate
• Concentration and reactivity of the nucleophile
• Effect of the solvent
• Nature of the leaving group
• Stereochemistry of SN2 Mechanism
Learning
Objectives
At the end of this
lecture, student will be able to
• Explain substitution reaction
• Classify nucleophile & leaving group
• Explain kinetics and mechanism involved in SN2 reaction
• Explain factors affecting rate of SN2 reaction
• List out the factors affecting rate of SN2 reaction
• Explain the stereochemistry of SN2
Substitution
Reaction
• Definition
Z + R X à R Z
+ X
• Aliphatic nucleophilic substitution reaction
• Components required
– Substrate
– Nucleophile
– Solvent
Nucleophile
• Definition
– Nucleus loving
– Electron rich
– Attacking reagents
• Types
– Negative nucleophile
– Neutral nucleophile
Examples
• Negative nucleophile: Cl‾, OH‾, CN‾, OR‾, H‾, X‾, SH‾
• Neutral nucleophile: NH3, H2O, ROH, RSH, ROR
Leaving
Group
• Definition
• Good leaving group
– Weak base
– Halide ion
• Leaving ability
among halogens
I‾ > Br‾ > Cl‾ >F‾
• Example
Mechanism
of Aliphatic Nucleophilic Substitution
• SN1 – substitution nucleophilic unimolecular
• SN2 – substitution nucleophilic bimolecular
SN2
Reaction
• Definition
– Bimolecular nucleophilic substitution
• Example
• Kinetics
– 2nd order kinetics
– Rate α [substrate] [nucleophile]
– Rate = k [CH3Br] [OH‾]
• Mechanism
– Backside attack
Factors
affecting the rates of SN2 reaction
• Structure of the substrate
• Concentration and reactivity of the nucleophile
• Effect of the solvent
• Nature of the leaving group
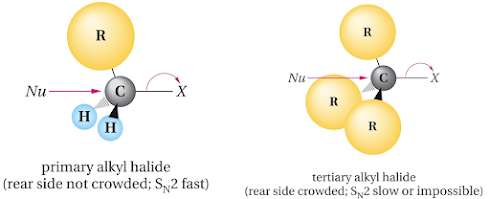
• Structure of the substrate
– Reactivity order
– Reason
• Steric hindrance
a. SN2 reaction of methyl bromide with hydroxide ion
b. SN2 reaction of sterically hindered alkyl halide with
hydroxide ion
• Concentration & strength of nucleophile
– Strong & high concentration of nucleophile
• Solvents
– Polar aprotic solvents
• Leaving group
– Good leaving groups
– Weak bases
– Halide ions
Role of solvents
• Polar protic solvents
– Decreases the rate of SN2 reaction
– Eact is high
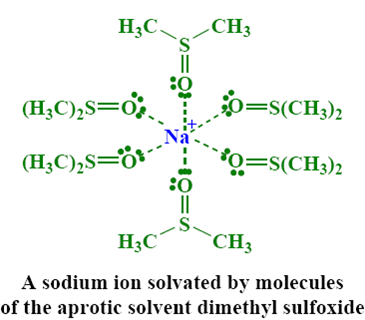
• Polar aprotic solvents
Increases the rate of SN2 reaction
– Solvate cations very well
– No hydrogen bonding
– Anions reactive as nucleophiles
• Polar aprotic solvents
Stereochemistry
of SN2
• Walden inversion
• Nucleophile attacks carbonfrom side opposite bond to the
leaving group
Inversion of
Configuration
Stereospecific
Reaction
• Stereoisomeric starting materials give stereoisomeric
products
• The reaction of 2-bromooctane with NaOH (in ethanol-water)
is stereospecific.
•
(+)-2-Bromooctane
(–)-2-Octanol
•
(–)-2-Bromooctane
(+)-2-Octanol
Summary
• Alkyl halides readily undergo nucleophilic substitution
reactions
• Nucleophile is electron rich, nucleus loving reagents
• Nucleophile substitutes for halogen which is the good
leaving group
• SN2 reaction is bimolecular – 2 molecules involved in the
rate determining step
• Nucleophile attacks the back side of the carbon that is
attached to the halogen
• Rate of SN2 reaction depends on steric hindrance
• Bulkier the groups at the backside of the carbon slower
the reaction
• Methyl halides and primary alkyl halides readily undergo
SN2 reaction
• Rate of SN2 reaction is favoured by strong & high
concentration of nucleophile in polar aprotic solvents
• SN2 reaction involves complete inversion of configuration
– Walden inversion
• Back side attack is preferred