Structure and Uses of Alcohols

Learning Objectives
At the end of this
lecture, student will be able to
• Write the structure and uses of alcohols
Structure and Uses of Alcohols
The structure of alcohols is characterized by the presence of the hydroxyl group (-OH), which consists of an oxygen atom bonded to a hydrogen atom. This hydroxyl group is attached to a carbon atom, and the carbon atom can be part of a linear or branched hydrocarbon chain. The type of alcohol is determined by the nature of the hydrocarbon group to which the hydroxyl group is attached.
Classification of Alcohols
Alcohols can be classified into three main categories based on the nature of the hydrocarbon group:
- Primary Alcohols: In primary alcohols, the carbon atom to which the hydroxyl group is attached is bonded to only one other carbon atom.
- Secondary Alcohols: Secondary alcohols have the hydroxyl group attached to a carbon atom bonded to two other carbon atoms.
- Tertiary Alcohols: Tertiary alcohols feature the hydroxyl group attached to a carbon atom bonded to three other carbon atoms.
The general structural formula for these three types of alcohols is as follows:
- Primary Alcohol: R-CH2OH
- Secondary Alcohol: R-CHR’-OH
- Tertiary Alcohol: R-CR’R”-OH
Here, R, R’, and R” represent hydrocarbon groups, which can be alkyl or aryl groups.
Uses of Alcohols
Alcohols have a wide range of applications in various industries and everyday life. Some of the most common uses of alcohols include:
1. Solvents
Alcohols are widely used as solvents in chemical processes and industries. They can dissolve a variety of organic and inorganic compounds, making them essential in the production of paints, varnishes, and pharmaceuticals.
2. Disinfectants and Antiseptics
Alcohols, particularly ethanol and isopropyl alcohol, are effective disinfectants and antiseptics. They are used for sanitizing surfaces, medical equipment, and as hand sanitizers to kill bacteria and viruses.
3. Fuel
Ethanol, a type of alcohol, is used as an alternative or additive in gasoline. It is considered a renewable and environmentally friendly fuel source.
4. Beverages
Alcoholic beverages like beer, wine, and spirits are made through the fermentation of sugars by yeast, resulting in the production of ethanol. These beverages are enjoyed worldwide for their recreational and social aspects.
5. Pharmaceuticals
Some alcohols are used in the pharmaceutical industry to formulate medications and drugs. They serve as carriers for certain active ingredients.
6. Cosmetics and Personal Care Products
Alcohols are commonly found in cosmetics and personal care products such as perfumes, lotions, and hair sprays. They act as carriers for fragrances and provide a quick-drying effect.
7. Chemical Synthesis
Alcohols are essential in various chemical reactions and syntheses, including the production of esters, ethers, and other organic compounds.
8. Preservatives
Alcohols are used as preservatives in the food industry to extend the shelf life of certain products. They inhibit the growth of bacteria and molds.
Ethyl alcohol
Structure of Ethyl alcohol
• Also called ethanol/ EtOH
Uses of Ethyl alcohol
• Antiseptic, methanol poisoning
• Good solvent in chemistry
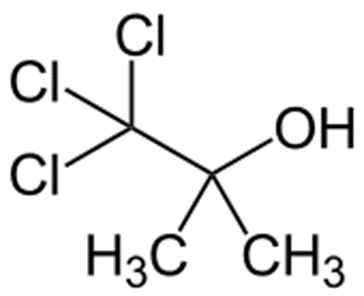
Chlorobutanol
Structure of Chlorobutanol
• IUPAC- trichloro-2-methyl-2-propanol
Uses of Chlorobutanol
• Sedative, hypnotic
• Preservative
• Weak local anaesthetic
• Antibacterial and antifungal
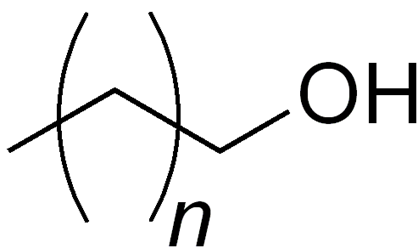
Cetosteryl alcohol
Structure of Cetosteryl alcohol
• Where n is ~14-16
• Also called as Cetostearyl alcohol, cetearyl alcohol or cetylstearyl alcohol, mixture of fatty alcohols
• Predominantly of cetyl (C16) and stearyl alcohols (C18)
Uses of Cetosteryl alcohol
• Emulsion stabilizer, opacifying agent, and foam boosting surfactant, viscosity increasing agent, emollient
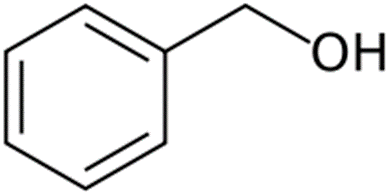
Benzyl alcohol
Structure of Benzyl alcohol
• Abbreviated as BnOH
Uses of Benzyl alcohol
• Solvent for inks, waxes, paints, resins
• Precursor for esters in soap, perfume and flavor industries
• 10% concentration- local anesthetic and antimicrobial
• Insect repellant
• Approved by USFDA- 5% soln. as bacteriostatic preservative
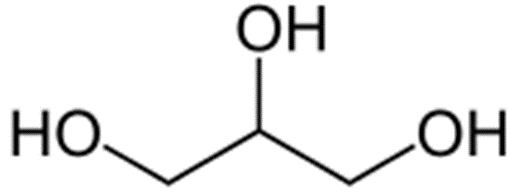
Glycerol
Structure of Glycerol
• Simple polyol compound- glycerine
Uses of Glycerol
• Food industry- sweetener
• Pharmaceutical- humectant, to improve smoothness, lubricant
• Laxative- suppository
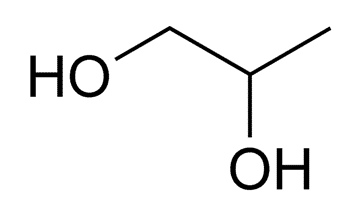
Propylene glycol
Structure of Propylene glycol
• IUPAC- propane-1,2-diol
Uses of Propylene glycol
• Production of polymers
• Humectant, good solvent
• In various edible items (food industry)
Summary
• Structures of different alcohols were studied
• Benzyl alcohol is insect repellent
• Propylene glycol is Humectant, good solvent
Also, Visit:
B. Pharma Notes | B. Pharma Notes | Study material Bachelor of Pharmacy pdf